Recurring homozygous ACTN2 variant (p.Arg506Gly) causes a recessive myopathy
- PMID: 38311799
- PMCID: PMC10963296
- DOI: 10.1002/acn3.51983
Recurring homozygous ACTN2 variant (p.Arg506Gly) causes a recessive myopathy
Abstract
Objective: ACTN2, encoding alpha-actinin-2, is essential for cardiac and skeletal muscle sarcomeric function. ACTN2 variants are a known cause of cardiomyopathy without skeletal muscle involvement. Recently, specific dominant monoallelic variants were reported as a rare cause of core myopathy of variable clinical onset, although the pathomechanism remains to be elucidated. The possibility of a recessively inherited ACTN2-myopathy has also been proposed in a single series.
Methods: We provide clinical, imaging, and histological characterization of a series of patients with a novel biallelic ACTN2 variant.
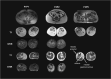
Results: We report seven patients from five families with a recurring biallelic variant in ACTN2: c.1516A>G (p.Arg506Gly), all manifesting with a consistent phenotype of asymmetric, progressive, proximal, and distal lower extremity predominant muscle weakness. None of the patients have cardiomyopathy or respiratory insufficiency. Notably, all patients report Palestinian ethnicity, suggesting a possible founder ACTN2 variant, which was confirmed through haplotype analysis in two families. Muscle biopsies reveal an underlying myopathic process with disruption of the intermyofibrillar architecture, Type I fiber predominance and atrophy. MRI of the lower extremities demonstrate a distinct pattern of asymmetric muscle involvement with selective involvement of the hamstrings and adductors in the thigh, and anterior tibial group and soleus in the lower leg. Using an in vitro splicing assay, we show that c.1516A>G ACTN2 does not impair normal splicing.
Interpretation: This series further establishes ACTN2 as a muscle disease gene, now also including variants with a recessive inheritance mode, and expands the clinical spectrum of actinopathies to adult-onset progressive muscle disease.
© 2024 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
KMc and AC are an employee of GeneDx, LLC. The remaining authors declare that they have no conflict of interest.
Figures




References
-
- Gautel M. The sarcomeric cytoskeleton: who picks up the strain? Curr Opin Cell Biol. 2011;23(1):39‐46. - PubMed
-
- Mohapatra B, Jimenez S, Lin JH, et al. Mutations in the muscle LIM protein and alpha‐actinin‐2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol Genet Metab. 2003;80(1‐2):207‐215. - PubMed
-
- Theis JL, Bos JM, Bartleson VB, et al. Echocardiographic‐determined septal morphology in Z‐disc hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2006;351(4):896‐902. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

