Updates on New Therapies for Patients with CKD
- PMID: 38312786
- PMCID: PMC10831355
- DOI: 10.1016/j.ekir.2023.10.006
Updates on New Therapies for Patients with CKD
Abstract
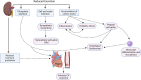
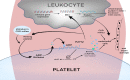
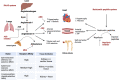
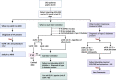
Individuals diagnosed with chronic kidney disease (CKD) continue to increase globally. This group of patients experience a disproportionately higher risk of cardiovascular (CV) events compared to the general population. Despite multiple guidelines-based medical management, patients with CKD continue to experience residual cardiorenal risk. Several potential mechanisms explain this excessive CV risk observed in individuals with CKD. Several new drugs have become available that could potentially transform CKD care, given their efficacy in this patient population. Nevertheless, use of these drugs presents certain benefits and challenges that are often underrecognized by prescribing these drugs. In this review, we aim to provide a brief discussion about CKD pathophysiology, limiting our discussion to recent published studies. We also explore benefits and limitations of newer drugs, including angiotensin receptor/neprilysin inhibitors (ARNI), sodium glucose transporter 2 inhibitors (SGLT2i), glucagon-like peptides-1 (GLP-1) agonists and finerenone in patients with CKD. Despite several articles covering this topic, our review provides an algorithm where subgroups of patients with CKD might benefit the most from such drugs based on the selection criteria of the landmark trials. Patients with CKD who have nephrotic range proteinuria beyond 5000 mg/g, or those with poorly controlled blood pressure (systolic ≥160 mm Hg or diastolic ≥100 mm Hg) remain understudied.
Keywords: ARNI; SGLT2; chronic kidney disease; finerenone; inflammation; platelets.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

