Multicenter Long-Term Real World Data on Treatment With Lumasiran in Patients With Primary Hyperoxaluria Type 1
- PMID: 38312792
- PMCID: PMC10831356
- DOI: 10.1016/j.ekir.2023.10.004
Multicenter Long-Term Real World Data on Treatment With Lumasiran in Patients With Primary Hyperoxaluria Type 1
Abstract
Introduction: The RNA interference (RNAi) medication lumasiran reduces hepatic oxalate production in primary hyperoxaluria type 1 (PH1). Data outside clinical trials are scarce.
Methods: We report on retrospectively and observationally obtained data in 33 patients with PH1 (20 with preserved kidney function, 13 on dialysis) treated with lumasiran for a median of 18 months.
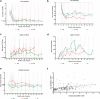
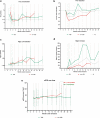
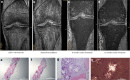
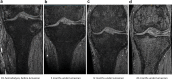
Results: Among those with preserved kidney function, mean urine oxalate (Uox) decreased from 1.88 (baseline) to 0.73 mmol/1.73 m2 per 24h after 3 months, to 0.72 at 12 months, and to 0.65 at 18 months, but differed according to vitamin B6 (VB6) medication. The highest response was at month 4 (0.55, -70.8%). Plasma oxalate (Pox) remained stable over time. Glomerular filtration rate increased significantly by 10.5% at month 18. Nephrolithiasis continued active in 6 patients, nephrocalcinosis ameliorated or progressed in 1 patient each. At last follow-up, Uox remained above 1.5 upper limit of normal (>0.75 mmol/1.73 m2 per 24h) in 6 patients. Urinary glycolate (Uglyc) and plasma glycolate (Pglyc) significantly increased in all, urine citrate decreased, and alkali medication needed adaptation. Among those on dialysis, mean Pox and Pglyc significantly decreased and increased, respectively after monthly dosing (Pox: 78-37.2, Pglyc: 216.4-337.4 μmol/l). At quarterly dosing, neither Pox nor Pglyc were significantly different from baseline levels. An acid state was buffered by an increased dialysis regimen. Systemic oxalosis remained unchanged.
Conclusion: Lumasiran treatment is safe and efficient. Dosage (interval) adjustment necessities need clarification. In dialysis, lack of Pox reduction may relate to dissolving systemic oxalate deposits. Pglyc increment may be a considerable acid load requiring careful consideration, which definitively needs further investigation.
Keywords: RNA interference; citrate; glycolate; oxalate; primary hyperoxaluria; treatment.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
LinkOut - more resources
Full Text Sources

