The role of ferroptosis in central nervous system damage diseases
- PMID: 38313006
- PMCID: PMC10836208
- DOI: 10.7717/peerj.16741
The role of ferroptosis in central nervous system damage diseases
Abstract
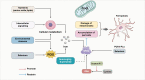
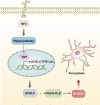
Ferroptosis is a form of cell death, i.e., programmed cell death characterized by lipid peroxidation and iron dependence, which has unique morphological and biochemical properties. This unique mode of cell death is driven by iron-dependent phospholipid peroxidation and regulated by multiple cell metabolic pathways, including redox homeostasis, iron metabolism, mitochondrial activity, and the metabolism of amino acids, lipids, and sugars. Many organ injuries and degenerative pathologies are caused by ferroptosis. Ferroptosis is closely related to central nervous system injury diseases and is currently an important topic of research globally. This research examined the relationships between ferroptosis and the occurrence and treatment of central nervous system injury diseases. Additionally, ferroptosis was assessed from the aspect of theory proposal, mechanism of action, and related signaling pathways per recent research. This review provides a relevant theoretical basis for further research on this theory, the prospect of its development, and the prevention and treatment of such diseases.
Keywords: Ferroptosis; GPX4; Nerve damage; Redox.
©2024 Li et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
Similar articles
-
Ferroptosis: A potential target for the intervention of intervertebral disc degeneration.Front Endocrinol (Lausanne). 2022 Oct 20;13:1042060. doi: 10.3389/fendo.2022.1042060. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36339421 Free PMC article. Review.
-
ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries.Int J Mol Sci. 2023 Jun 12;24(12):10021. doi: 10.3390/ijms241210021. Int J Mol Sci. 2023. PMID: 37373168 Free PMC article. Review.
-
Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis.Int J Mol Sci. 2022 Dec 27;24(1):449. doi: 10.3390/ijms24010449. Int J Mol Sci. 2022. PMID: 36613888 Free PMC article. Review.
-
Ferroptosis Is Regulated by Mitochondria in Neurodegenerative Diseases.Neurodegener Dis. 2020;20(1):20-34. doi: 10.1159/000510083. Epub 2020 Aug 19. Neurodegener Dis. 2020. PMID: 32814328 Review.
-
Ferroptosis and central nervous system demyelinating diseases.J Neurochem. 2023 Jun;165(6):759-771. doi: 10.1111/jnc.15831. Epub 2023 May 25. J Neurochem. 2023. PMID: 37095635 Review.
Cited by
-
Metformin inhibits the proliferation of hepatocellular carcinoma cells through inducing ferroptosis analyzed by phosphoproteomics.Front Oncol. 2025 May 30;15:1531420. doi: 10.3389/fonc.2025.1531420. eCollection 2025. Front Oncol. 2025. PMID: 40519291 Free PMC article.
-
The Interplay Between Endoplasmic Reticulum Stress and Ferroptosis in Neurological Diseases.Neurochem Res. 2025 Feb 10;50(2):99. doi: 10.1007/s11064-025-04348-4. Neurochem Res. 2025. PMID: 39928173 Review.
References
-
- Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA, Lao PJ, Stern Y, Vonsattel JP, Teich AF, Airey DC, Proctor NK, Dage JL, Mayeux R. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s & Dementia. 2021;17:1353–1364. doi: 10.1002/alz.12301. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

