Targeting VEGF using Bevacizumab attenuates sepsis-induced liver injury in a mouse model of cecal ligation and puncture
- PMID: 38313162
- PMCID: PMC10835558
- DOI: 10.25122/jml-2023-0064
Targeting VEGF using Bevacizumab attenuates sepsis-induced liver injury in a mouse model of cecal ligation and puncture
Abstract
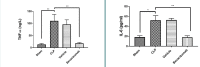
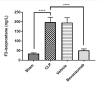
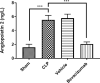
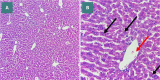
Sepsis, a life-threatening condition resulting from an uncontrolled host response to infection, often leads to severe liver damage and remains a significant cause of mortality in critically ill patients despite advances in antibiotic therapy and resuscitation. Bevacizumab, a neutralizing antibody targeting vascular endothelial growth factor (VEGF), is approved for treating certain cancers. However, its potential impact on sepsis-related liver injury is not well understood. This study aimed to explore the potential hepatoprotective effect of Bevacizumab on sepsis-induced liver injury. Twenty-four mice were divided into four groups: a sham group subjected to a midline incision only, a cecal ligation and puncture induction (CLP) group, a vehicle-treated group that received a vehicle one hour before CLP induction, and a Bevacizumab-treated group that received Bevacizumab one hour before CLP induction. Blood samples were collected, and angiopoietin-2 (ANGPT2), alanine transaminase (ALT), and aspartate transaminase (AST) serum levels were measured. Liver tissue homogenates were analyzed for IL-6, TNFα, intracellular adhesion molecule (ICAM-1), macrophage inhibitory factor (MIF), vascular endothelial growth factor (VEGF), F2-isoprostane, and caspase-11 levels. A histological examination was performed to assess the extent of liver damage. Mice exposed to CLP had high levels of the biomarkers mentioned above with a high degree of liver injury compared to the sham group. In contrast, treatment with Bevacizumab notably reduced these markers and mitigated liver damage. In conclusion, Bevacizumab may be a protective agent against sepsis-induced liver injury.
Keywords: Bevacizumab; VEGF; mouse model; sepsis-induced liver injury.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
