This is a preprint.
Exposure to Gulf war illness-related chemicals exacerbates alcohol- induced liver damage in rodents
- PMID: 38313276
- PMCID: PMC10836102
- DOI: 10.21203/rs.3.rs-3838282/v1
Exposure to Gulf war illness-related chemicals exacerbates alcohol- induced liver damage in rodents
Update in
-
Exposure to Gulf war illness-related chemicals exacerbates alcohol-induced liver damage in rodents.Sci Rep. 2024 Jul 1;14(1):14981. doi: 10.1038/s41598-024-65638-5. Sci Rep. 2024. PMID: 38951546 Free PMC article.
Abstract
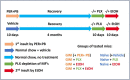
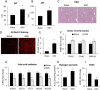
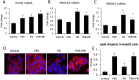
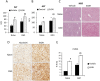
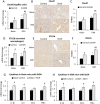
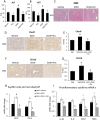
Gulf War Illness (GWI) describes a series of symptoms suffered by veterans of the Gulf war consisting of cognitive, neurological and gastrointestinal dysfunctions. Two chemicals associated with GWI are the insecticide permethrin (PER) and the nerve gas prophylactic pyridostigmine-bromide (PB). In this study we assessed the effects of PER and PB exposure on pathology and subsequent alcohol (EtOH)-induced liver injury, and the influence of a macrophage depletor, PLX3397, on EtOH-induced liver damage in PER/PB- treated mice. Male C57BL/6 mice were injected daily with vehicle or PER/PB for 10 days, followed by 4 months recovery, then treatment with PLX3397 and a chronic-plus-single-binge EtOH challenge for 10 days. PER/PB exposure resulted in the protracted increase in liver transaminases in the serum and induced chronic low-level microvesicular steatosis and inflammation in GWI vs Naïve mice up to 4 months after cessation of exposure. Furthermore, prior exposure to PER/PB also resulted in exacerbated response to EtOH-induced liver injury, with enhanced steatosis, ductular reaction and fibrosis. The enhanced EtOH-induced liver damage in GWI-mice was attenuated by strategies designed to deplete macrophages in the liver. Taken together, these data suggest that exposure to GWI-related chemicals may alter the liver's response to subsequent ethanol exposure.
Conflict of interest statement
Competing interests No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Similar articles
-
Exposure to Gulf war illness-related chemicals exacerbates alcohol-induced liver damage in rodents.Sci Rep. 2024 Jul 1;14(1):14981. doi: 10.1038/s41598-024-65638-5. Sci Rep. 2024. PMID: 38951546 Free PMC article.
-
Gulf war illness-related chemicals increase CD11b/c+ monocyte infiltration into the liver and aggravate hepatic cholestasis in a rodent model.Sci Rep. 2018 Sep 3;8(1):13147. doi: 10.1038/s41598-018-31599-9. Sci Rep. 2018. PMID: 30177688 Free PMC article.
-
Delayed treatment with the immunotherapeutic LNFPIII ameliorates multiple neurological deficits in a pesticide-nerve agent prophylactic mouse model of Gulf War Illness.Neurotoxicol Teratol. 2021 Sep-Oct;87:107012. doi: 10.1016/j.ntt.2021.107012. Epub 2021 Jul 10. Neurotoxicol Teratol. 2021. PMID: 34256162
-
Dysbiosis in gastrointestinal pathophysiology: Role of the gut microbiome in Gulf War Illness.J Cell Mol Med. 2023 Apr;27(7):891-905. doi: 10.1111/jcmm.17631. Epub 2023 Jan 30. J Cell Mol Med. 2023. PMID: 36716094 Free PMC article. Review.
-
Gulf War Illness: Unifying Hypothesis for a Continuing Health Problem.Int J Environ Res Public Health. 2019 Jan 3;16(1):111. doi: 10.3390/ijerph16010111. Int J Environ Res Public Health. 2019. PMID: 30609834 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

