This is a preprint.
Pathogenic autoantibody internalization in myositis
- PMID: 38313303
- PMCID: PMC10836124
- DOI: 10.1101/2024.01.15.24301339
Pathogenic autoantibody internalization in myositis
Update in
-
Pathological autoantibody internalisation in myositis.Ann Rheum Dis. 2024 Oct 21;83(11):1549-1560. doi: 10.1136/ard-2024-225773. Ann Rheum Dis. 2024. PMID: 38902010 Free PMC article.
Abstract
Objectives: Myositis is a heterogeneous family of autoimmune muscle diseases. As myositis autoantibodies recognize intracellular proteins, their role in disease pathogenesis has been unclear. This study aimed to determine whether myositis autoantibodies reach their autoantigen targets within muscle cells and disrupt the normal function of these proteins.
Methods: Confocal immunofluorescence microscopy was used to localize antibodies and other proteins of interest in myositis muscle biopsies. Bulk RNA sequencing was used to study the transcriptomic profiles of 668 samples from patients with myositis, disease controls, and healthy controls. Antibodies from myositis patients were introduced into cultured myoblasts by electroporation and the transcriptomic profiles of the treated myoblasts were studied by bulk RNA sequencing.
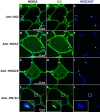
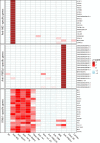
Results: In patients with myositis autoantibodies, antibodies accumulated inside myofibers in the same subcellular compartment as the autoantigen. Each autoantibody was associated with effects consistent with dysfunction of its autoantigen, such as the derepression of genes normally repressed by Mi2/NuRD in patients with anti-Mi2 autoantibodies, the accumulation of RNAs degraded by the nuclear RNA exosome complex in patients with anti-PM/Scl autoantibodies targeting this complex, and the accumulation of lipids within myofibers of anti-HMGCR-positive patients. Internalization of patient immunoglobulin into cultured myoblasts recapitulated the transcriptomic phenotypes observed in human disease, including the derepression of Mi2/NuRD-regulated genes in anti-Mi2-positive dermatomyositis and the increased expression of genes normally degraded by the nuclear RNA exosome complex in anti-PM/Scl-positive myositis.
Conclusions: In myositis, autoantibodies are internalized into muscle fibers, disrupt the biological function of their autoantigen, and mediate the pathophysiology of the disease.
Keywords: Myositis; RNA-sequencing; autoantibodies; dermatomyositis; immune-mediated necrotizing myositis.
Conflict of interest statement
Competing interests: None.
Figures



References
-
- Casal-Dominguez M, Pinal-Fernandez I, Pak K, et al. Performance of the 2017 European Alliance of Associations for Rheumatology/American College of Rheumatology Classification Criteria for Idiopathic Inflammatory Myopathies in Patients With Myositis-Specific Autoantibodies. Arthritis Rheumatol 2022;74(3):508–517. DOI: 10.1002/art.41964. - DOI - PMC - PubMed
-
- Arouche-Delaperche L, Allenbach Y, Amelin D, et al. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol 2017;81(4):538–548. DOI: 10.1002/ana.24902. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
