Ferroptosis in head and neck squamous cell carcinoma: from pathogenesis to treatment
- PMID: 38313306
- PMCID: PMC10834699
- DOI: 10.3389/fphar.2024.1283465
Ferroptosis in head and neck squamous cell carcinoma: from pathogenesis to treatment
Abstract
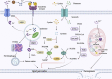
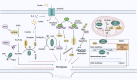
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignant tumor worldwide, with high morbidity and mortality. Surgery and postoperative chemoradiotherapy have largely reduced the recurrence and fatality rates for most HNSCCs. Nonetheless, these therapeutic approaches result in poor prognoses owing to severe adverse reactions and the development of drug resistance. Ferroptosis is a kind of programmed cell death which is non-apoptotic. Ferroptosis of tumor cells can inhibit tumor development. Ferroptosis involves various biomolecules and signaling pathways, whose expressions can be adjusted to modulate the sensitivity of cells to ferroptosis. As a tool in the fight against cancer, the activation of ferroptosis is a treatment that has received much attention in recent years. Therefore, understanding the molecular mechanism of ferroptosis in HNSCC is an essential strategy with therapeutic potential. The most important thing to treat HNSCC is to choose the appropriate treatment method. In this review, we discuss the molecular and defense mechanisms of ferroptosis, analyze the role and mechanism of ferroptosis in the inhibition and immunity against HNSCC, and explore the therapeutic strategy for inducing ferroptosis in HNSCC including drug therapy, radiation therapy, immunotherapy, nanotherapy and comprehensive treatment. We find ferroptosis provides a new target for HNSCC treatment.
Keywords: GSH; GXP4; ferroptosis; head and neck squamous cell carcinoma; pathogenesis; treatment.
Copyright © 2024 Yang and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

