Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 complex as a serum biomarker for COVID-19
- PMID: 38313435
- PMCID: PMC10835145
- DOI: 10.3389/fimmu.2023.1299792
Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 complex as a serum biomarker for COVID-19
Erratum in
-
Corrigendum: Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 complex as a serum biomarker for COVID-19.Front Immunol. 2024 Mar 13;15:1390698. doi: 10.3389/fimmu.2024.1390698. eCollection 2024. Front Immunol. 2024. PMID: 38545120 Free PMC article.
Abstract
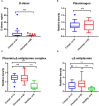
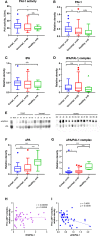
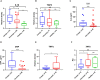
Patients with coronavirus disease-2019 (COVID-19) have an increased risk of thrombosis and acute respiratory distress syndrome (ARDS). Thrombosis is often attributed to increases in plasminogen activator inhibitor-1 (PAI-1) and a shut-down of fibrinolysis (blood clot dissolution). Decreased urokinase-type plasminogen activator (uPA), a protease necessary for cell-associated plasmin generation, and increased tissue-type plasminogen activator (tPA) and PAI-1 levels have been reported in COVID-19 patients. Because these factors can occur in free and complexed forms with differences in their biological functions, we examined the predictive impact of uPA, tPA, and PAI-1 in their free forms and complexes as a biomarker for COVID-19 severity and the development of ARDS. In this retrospective study of 69 Japanese adults hospitalized with COVID-19 and 20 healthy donors, we found elevated free, non-complexed PAI-1 antigen, low circulating uPA, and uPA/PAI-1 but not tPA/PAI-1 complex levels to be associated with COVID-19 severity and ARDS development. This biomarker profile was typical for patients in the complicated phase. Lack of PAI-1 activity in circulation despite free, non-complexed PAI-1 protein and plasmin/α2anti-plasmin complex correlated with suPAR and sVCAM levels, markers indicating endothelial dysfunction. Furthermore, uPA/PAI-1 complex levels positively correlated with TNFα, a cytokine reported to trigger inflammatory cell death and tissue damage. Those levels also positively correlated with lymphopenia and the pro-inflammatory factors interleukin1β (IL1β), IL6, and C-reactive protein, markers associated with the anti-viral inflammatory response. These findings argue for using uPA and uPA/PAI-1 as novel biomarkers to detect patients at risk of developing severe COVID-19, including ARDS.
Keywords: C-reactive protein; COVID-19; fibrinolysis; interleukin-6; plasminogen activator inhibitor-1; respiratory distress syndrome; thrombosis; urokinase-type plasminogen activator.
Copyright © 2024 Yatsenko, Rios, Nogueira, Takahashi, Tabe, Naito, Takahashi, Hattori and Heissig.
Conflict of interest statement
The authors TY, KH and BH have a patent pending for using uPA and uPA/PAI-1 complex measurements to determine disease severity in inflammatory diseases such as COVID-19 as demonstrated in this research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Comment on
-
Evaluation of soluble fibrin monomer complex in patients in SARS-CoV-2 COVID-19 infection-associated coagulopathy.Eur J Haematol. 2022 Apr;108(4):319-326. doi: 10.1111/ejh.13738. Epub 2022 Jan 10. Eur J Haematol. 2022. PMID: 34921683
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

