A Salt Bridge and Disulfide Bond within the Lassa Virus Fusion Domain Are Required for the Initiation of Membrane Fusion
- PMID: 38313535
- PMCID: PMC10831964
- DOI: 10.1021/acsomega.3c08632
A Salt Bridge and Disulfide Bond within the Lassa Virus Fusion Domain Are Required for the Initiation of Membrane Fusion
Abstract
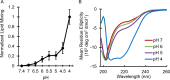
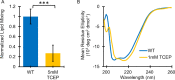
Infection with Lassa virus (LASV), an Old-World arenavirus that is endemic to West Africa, causes Lassa fever, a lethal hemorrhagic fever. Delivery of LASV's genetic material into the host cell is an integral component of its lifecycle. This is accomplished via membrane fusion, a process initiated by a hydrophobic sequence known as the fusion domain (FD). The LASV FD (G260-N295) consists of two structurally distinct regions: an N-terminal fusion peptide (FP: G260-T274) and an internal fusion loop (FL: C279-N295) that is connected by a short linker region (P275-Y278). However, the molecular mechanisms behind how the LASV FD initiates fusion remain unclear. Here, we demonstrate that the LASV FD adopts a fusogenic, helical conformation at a pH akin to that of the lysosomal compartment. Additionally, we identified a conserved disulfide bond (C279 and C292) and salt bridge (R282 and E289) within the FL that are pertinent to fusion. We found that the disulfide bond must be present so that the FD can bind to the lipid bilayer and subsequently initiate fusion. Moreover, the salt bridge is essential for the secondary structure of the FD such that it can associate with the lipid bilayer in the proper orientation for full functionality. In conclusion, our findings indicate that the LASV FD preferentially initiates fusion at a pH akin to that of the lysosome through a mechanism that requires a conserved salt bridge and, to a lesser extent, an intact disulfide bond within the internal FL.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
