The respiratory syncytial virus prefusion F protein vaccine attenuates the severity of respiratory syncytial virus-associated disease in breakthrough infections in adults ≥60 years of age
- PMID: 38314063
- PMCID: PMC10837780
- DOI: 10.1111/irv.13236
The respiratory syncytial virus prefusion F protein vaccine attenuates the severity of respiratory syncytial virus-associated disease in breakthrough infections in adults ≥60 years of age
Erratum in
-
Correction to: The Respiratory Syncytial Virus Prefusion F Protein Vaccine Attenuates the Severity of RSV-Associated Disease in Breakthrough Infections in Adults ≥60 Years of Age.Influenza Other Respir Viruses. 2024 Sep;18(9):e13364. doi: 10.1111/irv.13364. Influenza Other Respir Viruses. 2024. PMID: 39192516 Free PMC article. No abstract available.
-
Correction to "The Respiratory Syncytial Virus Prefusion F Protein Vaccine Attenuates the Severity of RSV-Associated Disease in Breakthrough Infections in Adults ≥60 Years of Age".Influenza Other Respir Viruses. 2025 Feb;19(2):e70088. doi: 10.1111/irv.70088. Influenza Other Respir Viruses. 2025. PMID: 39988966 Free PMC article. No abstract available.
Abstract
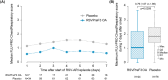
Background: Respiratory syncytial virus (RSV) is a contagious pathogen causing acute respiratory infections (ARIs). Symptoms range from mild upper respiratory tract infections to potentially life-threatening lower respiratory tract disease (LRTD). In adults ≥60 years old, vaccine efficacy of a candidate vaccine for older adults (RSVPreF3 OA) was 71.7% against RSV-ARI and 82.6% against RSV-LRTD (AReSVi-006/NCT04886596). We present the patient-reported outcomes (PROs) from the same trial at the end of the first RSV season in the northern hemisphere (April 2022).
Methods: In this phase 3 trial, adults aged ≥60 years were randomized (1:1) to receive one dose of RSVPreF3 OA vaccine or placebo. PROs were assessed using InFLUenza Patient-Reported Outcome (FLU-PRO), Short Form-12 (SF-12), and EuroQol-5 Dimension (EQ-5D) questionnaires. Peak FLU-PRO Chest/Respiratory scores during the first 7 days from ARI episode onset were compared using a Wilcoxon test. Least squares mean (LSMean) of SF-12 physical functioning (PF) and EQ-5D health utility scores were estimated using mixed effects models.
Results: In the RSVPreF3 OA group (N = 12,466), 27 first RSV-ARI episodes were observed versus 95 in the Placebo group (N = 12,494). Median peak FLU-PRO Chest/Respiratory scores were lower in RSVPreF3 OA (1.07) versus Placebo group (1.86); p = 0.0258. LSMean group differences for the PF and EQ-5D health utility score were 7.00 (95% confidence interval [CI]: -9.86, 23.85; p = 0.4125) and 0.0786 (95% CI: -0.0340, 0.1913; p = 0.1695).
Conclusions: The RSVPreF3 OA vaccine, in addition to preventing infection, attenuated the severity of RSV-associated symptoms in breakthrough infections, with trends of reduced impact on PF and health utility.
Keywords: acute respiratory infections; older adults; patient‐reported outcome; quality of life; respiratory syncytial virus.
© 2024 GSK. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
During this study, Eliazar Sabater Cabrera, Desmond Curran, Veronica Hulstrøm, Lusine Kostanyan, and Daniel Molnar were GSK employees. Eliazar Sabater Cabrera, Desmond Curran, Veronica Hulstrøm, Lusine Kostanyan, and Daniel Molnar hold shares in GSK. Isabel Maria Galan Melendez was an investigator for the study of RSV OA‐006 and declares to have received payments from GSK per contract, as well as equipment on loan and study materials. Silvia Narejos Pérez participated as principal investigator (PI) in clinical trials with different sponsors, including GSK and received financial support during the present manuscript. As a PI, she collaborated in the presentation of the results of clinical trials of vaccines and received financial support for attending meetings and/or travel. Laura Helman received payment for completing the study work and was given financial support for attending meetings and/or travel as an investigator. During the development of the study, John H. Powers III was a consultant for GSK and Vir on the clinical study design. Besides that, he received consulting fees from Adaptive Phage, Arrevus, Atheln, Bavarian Nordic, Cellularity, Eicos, Evofem, Eyecheck, Gilead, Mustang, OPKO, Otsuka, Resolve, Romark, Spine BioPharma, and UTIlity. He is an unpaid board member of Health Literacy Media. Mika Rämet declares that his institute was sponsored by GSK to perform the current study. Tino Schwarz has received honoraria for lecturing or advisory boards from Alexion, AstraZeneca, Bavarian Nordic, Biogen, Biontech, CSL Seqirus, GSK, Janssen‐Cilag, Merck‐Serono, Moderna, MSD, Novavax, Pfizer, Roche, Sanofi‐Aventis, and Takeda and for conducting clinical vaccine trials from GSK, Pfizer, Clover Biopharmaceuticals, and Serum Institute of India. Brigitte Stephan participated as investigator in clinical trials with different sponsors, including GSK. Sean Matthews, Lina Pérez Breva, Nathalie Roy, Dae Won Park, and Axel Schaefer have nothing to declare.
Figures

References
-
- Centers for Disease Control and Prevention . Transmission of RSV (respiratory syncytial virus). 2022. Accessed February 10, 2023. https://www.cdc.gov/rsv/about/transmission.html
-
- Centers for Disease Control and Prevention . RSV in older adults and adults with chronic medical conditions. 2022. Accessed February 10, 2023. https://www.cdc.gov/rsv/high-risk/older-adults.html
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

