SLC6A14 promotes ulcerative colitis progression by facilitating NLRP3 inflammasome-mediated pyroptosis
- PMID: 38314135
- PMCID: PMC10835529
- DOI: 10.3748/wjg.v30.i3.252
SLC6A14 promotes ulcerative colitis progression by facilitating NLRP3 inflammasome-mediated pyroptosis
Abstract
Background: Ulcerative colitis (UC) is an inflammatory condition with frequent relapse and recurrence. Evidence suggests the involvement of SLC6A14 in UC pathogenesis, but the central regulator remains unknown.
Aim: To explore the role of SLC6A14 in UC-associated pyroptosis.
Methods: Quantitative real-time polymerase chain reaction (qRT-PCR), immunoblotting, and immunohistochemical were used to assess SLC6A14 in human UC tissues. Lipopolysaccharide (LPS) was used to induce inflammation in FHC and NCM460 cells and model enteritis, and SLC6A14 levels were assessed. Pyroptosis markers were quantified using enzyme-linked immunosorbent assay, Western blotting, and qRT-PCR, and EdU incubation, CCK-8 assays and flow cytometry were used to examine proliferation and apoptosis. Mouse models of UC were used for verification.
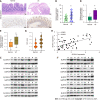
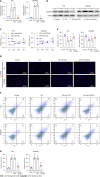
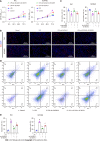
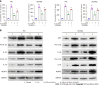
Results: SLC6A14 was increased and correlated with NLRP3 in UC tissues. LPS-induced FHC and NCM460 cells showed increased SLC6A14 levels. Reducing SLC6A14 increased cell proliferation and suppressed apoptosis. Reducing SLC6A14 decreased pyroptosis-associated proteins (ASC, IL-1β, IL-18, NLRP3). NLRP3 overexpression counteracted the effects of sh-SLC6A14 on LPS-induced FHC and NCM460 cell pyroptosis. SLC6A14 improved the mucosa in mice with dextran sulfate sodium-induced colitis.
Conclusion: SLC6A14 promotes UC pyroptosis by regulating NLRP3, suggesting the therapeutic potential of modulating the SLC6A14/NLRP3 axis.
Keywords: Inflammasome; NLRP3; Pyroptosis; SLC6A1; Ulcerative colitis.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures



Similar articles
-
PSMB5 Alleviates Ulcerative Colitis by Inhibiting ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis.Dis Markers. 2022 Aug 26;2022:2329904. doi: 10.1155/2022/2329904. eCollection 2022. Dis Markers. 2022. Retraction in: Dis Markers. 2023 Jul 19;2023:9827206. doi: 10.1155/2023/9827206. PMID: 36061354 Free PMC article. Retracted.
-
Oxymatrine Attenuates Ulcerative Colitis through Inhibiting Pyroptosis Mediated by the NLRP3 Inflammasome.Molecules. 2024 Jun 18;29(12):2897. doi: 10.3390/molecules29122897. Molecules. 2024. PMID: 38930963 Free PMC article.
-
MALAT1 promotes colonic epithelial cell apoptosis and pyroptosis by sponging miR-22-3p to enhance NLRP3 expression.PeerJ. 2024 Nov 18;12:e18449. doi: 10.7717/peerj.18449. eCollection 2024. PeerJ. 2024. PMID: 39575175 Free PMC article.
-
Emerging role of exosomes in ulcerative colitis: Targeting NOD-like receptor family pyrin domain containing 3 inflammasome.World J Gastroenterol. 2024 Feb 14;30(6):527-541. doi: 10.3748/wjg.v30.i6.527. World J Gastroenterol. 2024. PMID: 38463022 Free PMC article. Review.
-
Progress on Regulation of NLRP3 Inflammasome by Chinese Medicine in Treatment of Ulcerative Colitis.Chin J Integr Med. 2023 Aug;29(8):750-760. doi: 10.1007/s11655-023-3551-1. Epub 2023 Apr 29. Chin J Integr Med. 2023. PMID: 37148482 Review.
Cited by
-
A Comprehensive Review of Food-Derived Compounds Targeting Pyroptosis for Colitis Therapy: From Effects to Mechanisms.J Inflamm Res. 2025 Aug 27;18:11667-11688. doi: 10.2147/JIR.S531820. eCollection 2025. J Inflamm Res. 2025. PMID: 40896541 Free PMC article. Review.
-
SLC6A14 as a Key Diagnostic Biomarker for Ulcerative Colitis: An Integrative Bioinformatics and Machine Learning Approach.Biochem Genet. 2025 Jan 13. doi: 10.1007/s10528-025-11027-0. Online ahead of print. Biochem Genet. 2025. PMID: 39806040
-
GPR35 prevents osmotic stress induced cell damage.Commun Biol. 2025 Mar 22;8(1):478. doi: 10.1038/s42003-025-07848-9. Commun Biol. 2025. PMID: 40121360 Free PMC article.
-
Evaluating the significance of ECSCR in the diagnosis of ulcerative colitis and drug efficacy assessment.Front Immunol. 2024 Aug 7;15:1426875. doi: 10.3389/fimmu.2024.1426875. eCollection 2024. Front Immunol. 2024. PMID: 39170615 Free PMC article.
-
Modified Pulsatilla decoction alleviates 5-fluorouracil-induced intestinal mucositis by modulating the TLR4/MyD88/NF-κB pathway and gut microbiota.World J Gastroenterol. 2025 Feb 21;31(7):98806. doi: 10.3748/wjg.v31.i7.98806. World J Gastroenterol. 2025. PMID: 39991674 Free PMC article.
References
-
- Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:643–654. - PubMed
-
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. - PubMed
-
- Kaenkumchorn T, Wahbeh G. Ulcerative Colitis: Making the Diagnosis. Gastroenterol Clin North Am. 2020;49:655–669. - PubMed
-
- Eisenstein M. Ulcerative colitis: towards remission. Nature. 2018;563:S33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

