Mesenchymal stromal cells in myeloid malignancies: Immunotherapeutic opportunities
- PMID: 38314300
- PMCID: PMC10837636
- DOI: 10.1016/j.heliyon.2024.e25081
Mesenchymal stromal cells in myeloid malignancies: Immunotherapeutic opportunities
Abstract
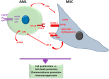
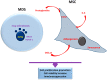
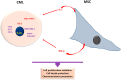
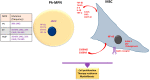
Myeloid malignancies are clonal disorders of the progenitor cells or hematopoietic stem cells, including acute myeloid leukemia, myelodysplastic syndromes, myeloproliferative malignancies, and chronic myelomonocytic leukemia. Myeloid neoplastic cells affect the proliferation and differentiation of other hematopoietic lineages in the bone marrow and peripheral blood, leading to severe and life-threatening complications. Mesenchymal stromal cells (MSCs) residing in the bone marrow exert immunosuppressive functions by suppressing innate and adaptive immune systems, thus creating a supportive and tolerant microenvironment for myeloid malignancy progression. This review summarizes the significant features of MSCs in myeloid malignancies, including their role in regulating cell growth, cell death, and antineoplastic resistance, in addition to their immunosuppressive contributions. Understanding the implications of MSCs in myeloid malignancies could pave the path for potential use in immunotherapy.
Keywords: Cell differentiation; Mesenchymal stromal cells; Myeloid cells; Myeloid malignancies; T-cell immunosuppression therapy.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Myeloid-Derived Suppressor Cells and Mesenchymal Stem/Stromal Cells in Myeloid Malignancies.J Clin Med. 2021 Jun 24;10(13):2788. doi: 10.3390/jcm10132788. J Clin Med. 2021. PMID: 34202907 Free PMC article. Review.
-
Myeloid-derived suppressor cells: Implication in myeloid malignancies and immunotherapy.Acta Histochem. 2024 Oct;126(5-7):152183. doi: 10.1016/j.acthis.2024.152183. Epub 2024 Jul 18. Acta Histochem. 2024. PMID: 39029317 Review.
-
Adipogenic Mesenchymal Stromal Cells from Bone Marrow and Their Hematopoietic Supportive Role: Towards Understanding the Permissive Marrow Microenvironment in Acute Myeloid Leukemia.Stem Cell Rev Rep. 2016 Apr;12(2):235-44. doi: 10.1007/s12015-015-9639-z. Stem Cell Rev Rep. 2016. PMID: 26649729
-
Mesenchymal stromal cells from myelodysplastic and acute myeloid leukemia patients display in vitro reduced proliferative potential and similar capacity to support leukemia cell survival.Stem Cell Res Ther. 2018 Oct 25;9(1):271. doi: 10.1186/s13287-018-1013-z. Stem Cell Res Ther. 2018. PMID: 30359303 Free PMC article.
-
Aging- and Senescence-associated Changes of Mesenchymal Stromal Cells in Myelodysplastic Syndromes.Cell Transplant. 2018 May;27(5):754-764. doi: 10.1177/0963689717745890. Epub 2018 Apr 23. Cell Transplant. 2018. PMID: 29682980 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

