Impact of Antihypertensive Medication Changes After Renal Denervation Among Different Patient Groups: SPYRAL HTN-ON MED
- PMID: 38314554
- PMCID: PMC11025607
- DOI: 10.1161/HYPERTENSIONAHA.123.22251
Impact of Antihypertensive Medication Changes After Renal Denervation Among Different Patient Groups: SPYRAL HTN-ON MED
Abstract
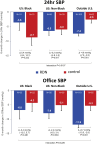
Background: The SPYRAL HTN-ON MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications)trial showed significant office and nighttime systolic blood pressure (BP) reductions in patients with hypertension following renal denervation (RDN) compared with sham-control patients, despite similar 24-hour BP reductions. We compared antihypertensive medication and BP changes among prespecified subpopulations.
Methods: The multicenter, randomized, sham-controlled, blinded SPYRAL HTN-ON MED trial (n=337) evaluated BP changes after RDN compared with a sham procedure in patients with hypertension prescribed 1 to 3 antihypertensive drugs. Most patients (n=187; 54%) were enrolled outside the United States, while 156 (46%) US patients were enrolled, including 60 (18%) Black Americans.
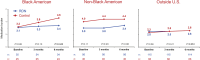
Results: Changes in detected antihypertensive drugs were similar between RDN and sham group patients in the outside US cohort, while drug increases were significantly more common in the US sham group compared with the RDN group. Patients from outside the United States showed significant reductions in office and 24-hour mean systolic BP at 6 months compared with the sham group, whereas BP changes were similar between RDN and sham in the US cohort. Within the US patient cohort, Black Americans in the sham control group had significant increases in medication burden from baseline through 6 months (P=0.003) but not in the RDN group (P=0.44).
Conclusions: Patients enrolled outside the United States had minimal antihypertensive medication changes between treatment groups and had significant office and 24-hour BP reductions compared with the sham group. Increased antihypertensive drug burden in the US sham cohort, especially among Black Americans, may have diluted the treatment effect in the combined trial population.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02439775.
Keywords: Black or African American; antihypertensive agents; blood pressure; hypertension; renal denervation.
Conflict of interest statement
Figures



References
-
- Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1 - PMC - PubMed
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

