The RIVET RCT: Rifamycin SV MMX improves muscle mass, physical function, and ammonia in cirrhosis and minimal encephalopathy
- PMID: 38315140
- PMCID: PMC10843468
- DOI: 10.1097/HC9.0000000000000384
The RIVET RCT: Rifamycin SV MMX improves muscle mass, physical function, and ammonia in cirrhosis and minimal encephalopathy
Abstract
Background: Minimal hepatic encephalopathy (MHE) negatively affects the prognosis of cirrhosis, but treatment is not standard. Rifamycin SV MMX (RiVM) is a nonabsorbable rifampin derivative with colonic action.
Methods: In a phase 2 placebo-controlled, double-blind randomized clinical trial patients with MHE were randomized to RiVM or placebo for 30 days with a 7-day follow-up. The primary endpoint was a change in stool cirrhosis dysbiosis ratio. Gut-brain (cognition, stool/salivary microbiome, ammonia, brain magnetic resonance spectroscopy), inflammation (stool calprotectin/serum cytokines), patient-reported outcomes (sickness impact profile: total/physical/psychosocial, high = worse), and sarcopenia (handgrip, bioelectric impedance) were secondary. Between/within groups and delta (post-pre) comparisons were performed.
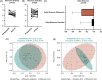
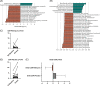
Results: Thirty patients (15/group) were randomized and completed the study without safety concerns. While cirrhosis dysbiosis ratio was statistically similar on repeated measures ANOVA (95% CI: -0.70 to 3.5), ammonia significantly reduced (95% CI: 4.4-29.6) in RiVM with changes in stool microbial α/β-diversity. MHE status was unchanged but only serial dotting (which tests motor strength) improved in RiVM-assigned patients. Delta physical sickness impact profile (95% CI: 0.33 = 8.5), lean mass (95% CI: -3.3 to -0.9), and handgrip strength (95% CI: -8.1 to -1.0) improved in RiVM versus placebo. Stool short-chain fatty acids (propionate, acetate, and butyrate) increased post-RiVM. Serum, urine, and stool bile acid profile changed to nontoxic bile acids (higher hyocholate/ursodeoxycholate and lower deoxycholate/lithocholate) post-RiVM. Serum IL-1β and stool calprotectin decreased while brain magnetic resonance spectroscopy showed higher glutathione concentrations in RiVM.
Conclusions: RiVM is well tolerated in patients with MHE with changes in stool microbial composition and function, ammonia, inflammation, brain oxidative stress, and sarcopenia-related parameters without improvement in cognition. RiVM modulates the gut-brain axis and gut-muscle axis in cirrhosis.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Jasmohan S. Bajaj’s institution gets research funding from Cosmo, Bausch, Grifols, and Sequana. Jasmohan S. Bajaj has consulted for Merz and Seres therapeutics. The remaining authors have no conflicts to report.
Figures



Comment in
-
Letter to the Editor: Rifamycin SV MMX was superior to placebo, but was the comparison appropriate?Hepatol Commun. 2024 Jul 18;8(8):e0470. doi: 10.1097/HC9.0000000000000470. eCollection 2024 Aug 1. Hepatol Commun. 2024. PMID: 39023341 Free PMC article. No abstract available.
References
-
- Gairing SJ, Mangini C, Zarantonello L, Gioia S, Nielsen EJ, Danneberg S, et al. . Prevalence of minimal hepatic encephalopathy in patients with liver cirrhosis: A multicenter study. Am J Gastroenterol. 2023;118:2191–2200. - PubMed
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. . Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. - PubMed
-
- Acharya C, Bajaj JS. Current management of hepatic encephalopathy. Am J Gastroenterol. 2018;113:1600–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

