Precision treatment of viral pneumonia through macrophage-targeted lipid nanoparticle delivery
- PMID: 38315853
- PMCID: PMC10873611
- DOI: 10.1073/pnas.2314747121
Precision treatment of viral pneumonia through macrophage-targeted lipid nanoparticle delivery
Abstract
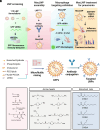
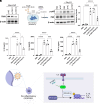
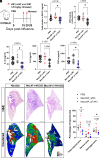
Macrophages are integral components of the innate immune system, playing a dual role in host defense during infection and pathophysiological states. Macrophages contribute to immune responses and aid in combatting various infections, yet their production of abundant proinflammatory cytokines can lead to uncontrolled inflammation and worsened tissue damage. Therefore, reducing macrophage-derived proinflammatory cytokine release represents a promising approach for treating various acute and chronic inflammatory disorders. However, limited macrophage-specific delivery vehicles have hindered the development of macrophage-targeted therapies. In this study, we screened a pool of 112 lipid nanoparticles (LNPs) to identify an optimal LNP formulation for efficient siRNA delivery. Subsequently, by conjugating the macrophage-specific antibody F4/80 to the LNP surface, we constructed MacLNP, an enhanced LNP formulation designed for targeted macrophage delivery. In both in vitro and in vivo experiments, MacLNP demonstrated a significant enhancement in targeting macrophages. Specifically, delivery of siRNA targeting TAK1, a critical kinase upstream of multiple inflammatory pathways, effectively suppressed the phosphorylation/activation of NF-kB. LNP-mediated inhibition of NF-kB, a key upstream regulator in the classic inflammatory signaling pathway, in the murine macrophage cell line RAW264.7 significantly reduced the release of proinflammatory cytokines after stimulation with the viral RNA mimic Poly(I:C). Finally, intranasal administration of MacLNP-encapsulated TAK1 siRNA markedly ameliorated lung injury induced by influenza infection. In conclusion, our findings validate the potential of targeted macrophage interventions in attenuating inflammatory responses, reinforcing the potential of LNP-mediated macrophage targeting to treat pulmonary inflammatory disorders.
Keywords: RNAi therapeutics; inhalation delivery; lipid nanoparticles; nanomedicine; pneumonia.
Conflict of interest statement
Competing interests statement:A.E.V., M.J.M., G.Z., and L.X. have filed a patent application based on this study. The other authors declare no competing interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

