Biomarker analysis of the ASPEN study comparing zanubrutinib with ibrutinib for patients with Waldenström macroglobulinemia
- PMID: 38315878
- PMCID: PMC11006814
- DOI: 10.1182/bloodadvances.2023010906
Biomarker analysis of the ASPEN study comparing zanubrutinib with ibrutinib for patients with Waldenström macroglobulinemia
Abstract
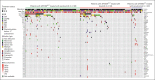
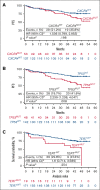
The phase 3 ASPEN trial (NCT03053440) compared Bruton tyrosine kinase inhibitors (BTKis), zanubrutinib and ibrutinib, in patients with Waldenström macroglobulinemia (WM). Post-hoc biomarker analysis was performed using next-generation sequencing on pretreatment bone marrow samples from 98 patients treated with zanubrutinib and 92 patients treated with ibrutinib with mutated (MUT) MYD88 and 20 patients with wild-type (WT) MYD88 treated with zanubrutinib. Of 329 mutations in 52 genes, mutations in CXCR4 (25.7%), TP53 (24.8%), ARID1A (15.7%), and TERT (9.0%) were most common. TP53MUT, ARID1AMUT, and TERTMUT were associated with higher rates of CXCR4MUT (P < .05). Patients with CXCR4MUT (frameshift or nonsense [NS] mutations) had lower very good partial response (VGPR) and complete response rates (CR; 17.0% vs 37.2%, P = .020) and longer time to response (11.1 vs 8.4 months) than patients with CXCR4WT treated with BTKis. CXCR4NS was associated with inferior progression-free survival (PFS; hazard ratio [HR], 3.39; P = .017) in patients treated with ibrutinib but not in those treated with zanubrutinib (HR, 0.67; P = .598), but VGPR + CR rates were similar between treatment groups (14.3% vs 15.4%). Compared with ibrutinib, patients with CXCR4NS treated with zanubrutinib had a favorable major response rate (MRR; 85.7% vs 53.8%; P = .09) and PFS (HR, 0.30; P = .093). In patients with TP53MUT, significantly lower MRRs were observed for patients treated with ibrutinib (63.6% vs 85.7%; P = .04) but not for those treated with zanubrutinib (80.8% vs 81.9%; P = .978). In TP53MUT, compared with ibrutinib, patients treated with zanubrutinib had higher VGPR and CR (34.6% vs 13.6%; P < .05), numerically improved MRR (80.8% vs 63.6%; P = .11), and longer PFS (not reached vs 44.2 months; HR, 0.66; P = .37). Collectively, patients with WM with CXCR4MUT or TP53MUT had worse prognosis compared with patients with WT alleles, and zanubrutinib led to better clinical outcomes.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.S.T. receives research funding from Janssen, AbbVie, and BeiGene; and receives honoraria from Janssen, AbbVie, BeiGene, LOXO, and AstraZeneca. S.D. is an employee of, and has equity ownership in, BeiGene; has acted as a consultant/advisor for Janssen, BeiGene, and Sanofi; and has received research funding from BeiGene. W.J. has acted as a consultant/advisor for AbbVie, AstraZeneca, BeiGene, Lilly, Roche, and Takeda; and has received research funding from AbbVie, AstraZeneca, Janssen, BeiGene, Lilly, Roche, Merck, MorphoSys, and Takeda. H.-P.L. has acted as a consultant/advisor for BeiGene. G.C. has received research funding from BeiGene, AstraZeneca, and Glycomimetics. R.G.O. has received honoraria from Janssen-Cilag, BeiGene, and AstraZeneca; and has acted as a consultant/advisor for BeiGene and Janssen-Cilag. P.M. has received consulting fees from BeiGene, Janssen, Astellas, AbbVie, Roche, Novartis, Gilead, IQVIA, and Otsuka; has received honoraria from Janssen, AbbVie, and Roche; and has served on an advisory board for The Australasian Leukaemia and Lymphoma Group. B.E.W. has received research funding from Roche and Incyte. R.G.-S. has received research funding from Gilead and Astellas; has received loyalties from IVS; has received consulting fees from Janssen, Incyte, and BeiGene; and has received honoraria from Millenium/Takeda, Janssen, Incyte, Amgen, BeiGene, AstraZeneca, and Pfizer. H.M. has received honoraria from AstraZeneca, BeiGene, and Janssen. A.T. has received honoraria from Janssen, AbbVie, AstraZeneca, and BeiGene; and has received travel support from Janssen, AbbVie, BeiGene, and AstraZeneca. J.J.C. has acted as a consultant/advisor for AbbVie, BeiGene, Cellectar, Janssen, and PharmaCytics; and has received research funding from AbbVie, AstraZeneca, BeiGene, Loxo Oncology, Pharmacyclics, and TG Therapeutics. J.C. has received travel expenses from Janssen. C.F.D.L.R. has received honoraria from Janssen, BeiGene, Bristol Myers Squibb, Amgen, and GlaxoSmithKline; has acted as a consultant/advisor for Janssen, BeiGene, Bristol Myers Squibb, Amgen, GlaxoSmithKline, and Pfizer; has received research funding from Janssen, Amgen, and Bristol Myers Squibb; and has received travel expenses from Janssen, BeiGene, Bristol Myers Squibb, Amgen, and GlaxoSmithKline. D.B. has acted as a consultant/advisor for Roche, Takeda, Janssen-Cilag, Gilead Sciences, and Novartis; has received research funding from Roche, Janssen-Cilag, Genmab, and MorphoSys; and has received travel expenses from Roche, Takeda, and Gilead Sciences. E.L. has acted as a consultant/advisor for Pharmacyclics; and has received research funding from BeiGene, Bristol Myers Squibb, and Janssen. J.M. has received consulting fees from BeiGene and Pharmacyclics; has received honoraria from BeiGene; and has participated in a data safety monitoring board or advisory board for BeiGene. M. Trněný has received honoraria from Takeda, Novartis, Janssen, AbbVie, Gilead Sciences, Roche, Bristol Myers Squibb, Amgen, and MorphoSys; has acted as a consultant/advisor for Roche, Bristol Myers Squibb, Amgen, Gilead Sciences, Novartis, MorphoSys, Incyte, Takeda, AbbVie, and Janssen; and has received travel funding from Gilead Sciences, Bristol Myers Squibb, Janssen, Takeda, Roche, and AbbVie. M.C.M. has acted as a consultant/advisor for Janssen-Cilag; and has served on the speaker’s bureau for Bristol Myers Squibb, Janssen-Cilag, and Medscape. C.B. has received honoraria from Roche/Genentech, Janssen, BeiGene, Novartis, Pfizer, Incyte, AbbVie, Gilead Sciences, Celltrion, MorphoSys, Regeneron, and Sobi; has received research funding from Roche/Genentech, Janssen, Celltrion, Merck Sharp & Dohme, Pfizer, and Amgen; and has served on the speaker’s bureau for Roche/Genentech, Janssen, BeiGene, Novartis, Pfizer, Incyte, AbbVie, Gilead Sciences, Celltrion, MorphoSys, Regeneron, Sobi, and Lilly. V.L. has received consulting fees from BeiGene, AstraZeneca, and Merck Sharp & Dohme. S.P.T. has received research funding from BeiGene; has received consulting fees from BeiGene, Janssen, and Pharmacyclics/AbbVie; and has received honoraria from BeiGene, Pharmacyclics/AbbVie, and Janssen. J.T. has served on an advisory board for BeiGene. W.Y.C. has equity in Bristol Myers Squibb. M.D. has received honoraria from BeiGene, Amgen, Bristol Myers Squibb, Takeda, and Janssen. B.W., Y.Y., Z.S., W.Y.C., J.S., H.A., and A.C. are employees of, and have equity ownership in, BeiGene. The remaining authors declare no competing financial interests.
Figures

References
-
- Gertz MA. Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94(2):266–276. - PubMed
-
- Hunter ZR, Xu L, Yang G, et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123(11):1637–1646. - PubMed
-
- Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood. 2013;122(7):1222–1232. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

