Efficacy and safety of novel left ventricular pacing leads: 1-year analysis of the NAVIGATOR trial
- PMID: 38316493
- PMCID: PMC10860098
- DOI: 10.1136/openhrt-2023-002517
Efficacy and safety of novel left ventricular pacing leads: 1-year analysis of the NAVIGATOR trial
Abstract
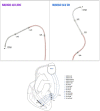
Objectives: Assess safety and performance of novel quadripolar preshaped left ventricular (LV) leads: NAVIGO 4LV 2D ('S shaped') and NAVIGO 4LV ARC ('U shaped').
Methods: Patients indicated for cardiac resynchronisation therapy were enrolled in a multicentre, prospective, controlled study (NAVIGATOR, NCT03279484). Patients were implanted with either a NAVIGO 4LV 2D or ARC lead, and assessed at 10 weeks, 6, 12 and 24 months post-implant. Co-primary safety and performance endpoints were assessed at 10 weeks. Safety endpoint was the patients' rate free from lead-related complications. Performance endpoint was the rate of patients with successful lead performance, defined as LV pacing threshold ≤2.5 V at 0.5 ms on at least one pacing vector, and the absence of phrenic nerve stimulation at the final programmed configuration. Lead-related complications and electrical parameters were monitored throughout study.
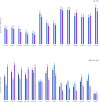
Results: A NAVIGO 4LV lead was successfully implanted in 211 out of 217 patients (97.2%). The safety endpoint was met, with 100% and 96.1% of patients free from complications for NAVIGO 4LV 2D and ARC, respectively. The performance endpoint was met with 98.1% and 98.9% of patients with a successful lead performance for NAVIGO 4LV 2D and ARC, respectively. Over 12 months, the global complication-free rate for both leads was 97.1% (95% CI: 93.71% to 98.70%), with a mean pacing capture threshold of 1.23 V±0.73 V and a mean impedance of 951 Ω±300.1 Ω.
Conclusion: A high implantation success rate and low complication rate was reported for the novel NAVIGO 4LV 2D and ARC leads, along with successful performance up to 12 months.
Keywords: LV lead; cardiac resynchronization therapy; complication; safety.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ET: Honoraria from MicroPort CRM. CI: MicroPort CRM Honoraria for presentations, reimbursement for travel/congress costs, participation in this sponsored research activity. MO: MicroPort CRM Honoraria for presentations.
Figures
References
-
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology/American heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and Antiarrhythmia devices) developed in collaboration with the American Association for Thoracic surgery and society of Thoracic Surgeons. J Am Coll Cardiol 2008;51:e1–62. 10.1016/j.jacc.2008.02.032 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
