Metastatic gastric cancer target lesion complete response with Claudin18.2-CAR T cells
- PMID: 38316518
- PMCID: PMC10860094
- DOI: 10.1136/jitc-2023-007927
Metastatic gastric cancer target lesion complete response with Claudin18.2-CAR T cells
Abstract
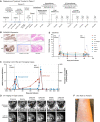
Treatment of hematologic malignancies with patient-derived anti-CD19 chimeric antigen receptor (CAR) T-cells has demonstrated long-term remissions for patients with otherwise treatment-refractory advanced leukemia and lymphoma. Conversely, CAR T-cell treatment of solid tumors, including advanced gastric cancer (GC), has proven more challenging due to on-target off-tumor toxicities, poor tumor T-cell infiltration, inefficient CAR T-cell expansion, immunosuppressive tumor microenvironments, and demanding preconditioning regimens. We report the exceptional results of autologous Claudin18.2-targeted CAR T cells (CT041) in a patient with metastatic GC, who had progressed on four lines of combined systemic chemotherapy and immunotherapy. After two CT041 infusions, the patient had target lesion complete response and sustained an 8-month overall partial response with only minimal ascites. Moreover, tumor-informed circulating tumor DNA (ctDNA) reductions coincided with rapid CAR T-cell expansion and radiologic response. No severe toxicities occurred, and the patient's quality of life significantly improved. This experience supports targeting Claudin18.2-positive GC with CAR T-cell therapy and helps to validate ctDNA as a biomarker in CAR T-cell therapy. Clinical Insight: Claudin18.2-targeted CAR T cells can safely provide complete objective and ctDNA response in salvage metastatic GC.
Keywords: Gastrointestinal Neoplasms; Receptors, Chimeric Antigen; Therapies, Investigational; Translational Medical Research.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HM, GS, JJ, AYH, JVNF and ZL are employees of CARsgen Therapeutics Corporation.
Figures

References
-
- Stomach cancer — cancer STAT facts. n.d. Available: https://seer.cancer.gov/statfacts/html/stomach.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
