Transcranial Low-Intensity Focused Ultrasound Stimulation of the Visual Thalamus Produces Long-Term Depression of Thalamocortical Synapses in the Adult Visual Cortex
- PMID: 38316559
- PMCID: PMC10941064
- DOI: 10.1523/JNEUROSCI.0784-23.2024
Transcranial Low-Intensity Focused Ultrasound Stimulation of the Visual Thalamus Produces Long-Term Depression of Thalamocortical Synapses in the Adult Visual Cortex
Abstract
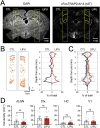
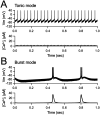
Transcranial focused ultrasound stimulation (tFUS) is a noninvasive neuromodulation technique, which can penetrate deeper and modulate neural activity with a greater spatial resolution (on the order of millimeters) than currently available noninvasive brain stimulation methods, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). While there are several studies demonstrating the ability of tFUS to modulate neuronal activity, it is unclear whether it can be used for producing long-term plasticity as needed to modify circuit function, especially in adult brain circuits with limited plasticity such as the thalamocortical synapses. Here we demonstrate that transcranial low-intensity focused ultrasound (LIFU) stimulation of the visual thalamus (dorsal lateral geniculate nucleus, dLGN), a deep brain structure, leads to NMDA receptor (NMDAR)-dependent long-term depression of its synaptic transmission onto layer 4 neurons in the primary visual cortex (V1) of adult mice of both sexes. This change is not accompanied by large increases in neuronal activity, as visualized using the cFos Targeted Recombination in Active Populations (cFosTRAP2) mouse line, or activation of microglia, which was assessed with IBA-1 staining. Using a model (SONIC) based on the neuronal intramembrane cavitation excitation (NICE) theory of ultrasound neuromodulation, we find that the predicted activity pattern of dLGN neurons upon sonication is state-dependent with a range of activity that falls within the parameter space conducive for inducing long-term synaptic depression. Our results suggest that noninvasive transcranial LIFU stimulation has a potential for recovering long-term plasticity of thalamocortical synapses in the postcritical period adult brain.
Keywords: LIFU; adult plasticity; long-term depression; noninvasive; synaptic plasticity; ultrasound.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Coleman JE, Nahmani M, Gavornik JP, Haslinger R, Heynen AJ, Erisir A, Bear MF (2010) Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci 30:9670–9682. 10.1523/JNEUROSCI.1248-10.2010 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
