Oral Probiotic Supplementation in Pregnancy to Reduce Group B Streptococcus Colonisation (OPSiP trial): study protocol for a double-blind parallel group randomised placebo trial
- PMID: 38316588
- PMCID: PMC10860072
- DOI: 10.1136/bmjopen-2023-076455
Oral Probiotic Supplementation in Pregnancy to Reduce Group B Streptococcus Colonisation (OPSiP trial): study protocol for a double-blind parallel group randomised placebo trial
Abstract
Introduction: Group B streptococcus (GBS), or Streptococcus agalactiae, remains a leading cause of neonatal morbidity and mortality. Canadian guidelines advise universal maternal screening for GBS colonisation in pregnancy in conjunction with selective antibiotic therapy. This results in over 1000 pregnant individuals receiving antibiotic therapy to prevent one case of early-onset neonatal GBS disease, and over 20 000 pregnant individuals receiving antibiotic therapy to prevent one neonatal death. Given the growing concern regarding the risk of negative sequela from antibiotic exposure, it is vital that alternative approaches to reduce maternal GBS colonisation are explored.Preliminary studies suggest some probiotic strains could confer protection in pregnancy against GBS colonisation.
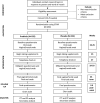
Methods and analysis: This double-blind parallel group randomised trial aims to recruit 450 pregnant participants in Vancouver, BC, Canada and will compare GBS colonisation rates in those who have received a daily oral dose of three strains of probiotics with those who have received a placebo. The primary outcome will be GBS colonisation status, measured using a vaginal/rectal swab obtained between 35 weeks' gestation and delivery. Secondary outcomes will include maternal antibiotic exposure and urogenital infections. Analysis will be on an intention-to-treat basis.
Patient or public involvement: There was no patient or public involvement in the design of the study protocol.
Ethics and dissemination: This study protocol received ethics approval from the University of British Columbia's Clinical Research Ethics Board, Dublin City University and Health Canada. Findings will be presented at research rounds, conferences and in peer-reviewed publications.
Trial registration number: NCT03407157.
Keywords: BACTERIOLOGY; Clinical Trial; INFECTIOUS DISEASES; Infections disease; Microbiology; NEONATOLOGY; OBSTETRICS; Pregnancy; Streptococcus agalactiae; probiotics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Ligilactobacillus salivarius V4II-90 eradicates Group B Streptococcus colonisation during pregnancy: a randomised, double-blind, placebo-controlled trial.Benef Microbes. 2024 Jun 28;15(4):387-396. doi: 10.1163/18762891-bja00021. Benef Microbes. 2024. PMID: 38955352 Clinical Trial.
-
Rapid intrapartum test for maternal group B streptococcal colonisation and its effect on antibiotic use in labouring women with risk factors for early-onset neonatal infection (GBS2): cluster randomised trial with nested test accuracy study.BMC Med. 2022 Jan 14;20(1):9. doi: 10.1186/s12916-021-02202-2. BMC Med. 2022. PMID: 35027057 Free PMC article. Clinical Trial.
-
Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial.J Matern Fetal Neonatal Med. 2021 Jun;34(11):1814-1821. doi: 10.1080/14767058.2019.1650907. Epub 2019 Aug 13. J Matern Fetal Neonatal Med. 2021. PMID: 31362572 Clinical Trial.
-
Preventing neonatal group B streptococcal infection. Intrapartum antibiotic prophylaxis in some high-risk situations.Prescrire Int. 2011 Mar;20(114):72-7. Prescrire Int. 2011. PMID: 21648230 Review.
-
The prevention of early-onset neonatal group B streptococcal disease.J Obstet Gynaecol Can. 2013 Oct;35(10):939-948. doi: 10.1016/S1701-2163(15)30818-5. J Obstet Gynaecol Can. 2013. PMID: 24165063 Review.
Cited by
-
Maternal and infant microbiome and birth anthropometry.iScience. 2024 Jun 21;27(10):110312. doi: 10.1016/j.isci.2024.110312. eCollection 2024 Oct 18. iScience. 2024. PMID: 39386758 Free PMC article.
-
SPIRIT 2025 explanation and elaboration: updated guideline for protocols of randomised trials.BMJ. 2025 Apr 28;389:e081660. doi: 10.1136/bmj-2024-081660. BMJ. 2025. PMID: 40294956 Free PMC article.
References
-
- Verani JR, McGee L, Schrag SJ, et al. . Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
