Reconfigurable droplet networks
- PMID: 38316759
- PMCID: PMC10844234
- DOI: 10.1038/s41467-024-45214-1
Reconfigurable droplet networks
Abstract
Droplet networks stabilized by lipid interfacial bilayers or colloidal particles have been extensively investigated in recent years and are of great interest for compartmentalized reactions and biological functions. However, current design strategies are disadvantaged by complex preparations and limited droplet size. Here, by using the assembly and jamming of cucurbit[8]uril surfactants at the oil-water interface, we show a novel means of preparing droplet networks that are multi-responsive, reconfigurable, and internally connected over macroscopic distances. Openings between the droplets enable the exchange of matter, affording a platform for chemical reactions and material synthesis. Our work requires only a manual compression to construct complex patterns of droplet networks, underscoring the simplicity of this strategy and the range of potential applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
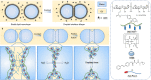

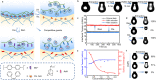

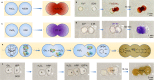

References
Grants and funding
- 52322310/National Natural Science Foundation of China (National Science Foundation of China)
- 52173018/National Natural Science Foundation of China (National Science Foundation of China)
- 22372005/National Natural Science Foundation of China (National Science Foundation of China)
- 2222071/Natural Science Foundation of Beijing Municipality (Beijing Natural Science Foundation)

