IL-17 promotes osteoclast-induced bone loss by regulating glutamine-dependent energy metabolism
- PMID: 38316760
- PMCID: PMC10844210
- DOI: 10.1038/s41419-024-06475-2
IL-17 promotes osteoclast-induced bone loss by regulating glutamine-dependent energy metabolism
Abstract
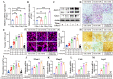
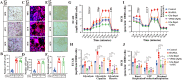
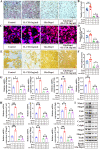
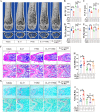
Osteoclasts consume an amount of adenosine triphosphate (ATP) to perform their bone resorption function in the development of osteoporosis. However, the mechanism underlying osteoclast energy metabolism has not been fully elucidated. In addition to glucose, glutamine (Glu) is another major energy carrier to produce ATP. However, the role of Glu metabolism in osteoclasts and the related molecular mechanisms has been poorly elucidated. Here we show that Glu is required for osteoclast differentiation and function, and that Glu deprivation or pharmacological inhibition of Glu transporter ASCT2 by V9302 suppresses osteoclast differentiation and their bone resorptive function. In vivo treatment with V9302 improved OVX-induced bone loss. Mechanistically, RNA-seq combined with in vitro and in vivo experiments suggested that Glu mediates the role of IL-17 in promoting osteoclast differentiation and in regulating energy metabolism. In vivo IL-17 treatment exacerbated OVX-induced bone loss, and this effect requires the participation of Glu or its downstream metabolite α-KG. Taken together, this study revealed a previously unappreciated regulation of IL-17 on energy metabolism, and this regulation is Glu-dependent. Targeting the IL-17-Glu-energy metabolism axis may be a potential therapeutic strategy for the treatment of osteoporosis and other IL-17 related diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dong Y, Zhang Y, Song K, Kang H, Ye D, Li F. What was the epidemiology and global burden of disease of hip fractures from 1990 to 2019? results from and additional analysis of the global burden of disease study 2019. Clin Orthop Related Res. 2023;481:1209–20. doi: 10.1097/CORR.0000000000002465. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

