Bystander activated CD8+ T cells mediate neuropathology during viral infection via antigen-independent cytotoxicity
- PMID: 38316762
- PMCID: PMC10844499
- DOI: 10.1038/s41467-023-44667-0
Bystander activated CD8+ T cells mediate neuropathology during viral infection via antigen-independent cytotoxicity
Abstract
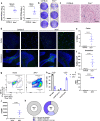
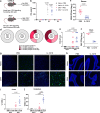
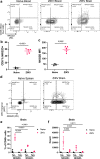
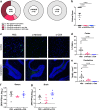
Although many viral infections are linked to the development of neurological disorders, the mechanism governing virus-induced neuropathology remains poorly understood, particularly when the virus is not directly neuropathic. Using a mouse model of Zika virus (ZIKV) infection, we found that the severity of neurological disease did not correlate with brain ZIKV titers, but rather with infiltration of bystander activated NKG2D+CD8+ T cells. Antibody depletion of CD8 or blockade of NKG2D prevented ZIKV-associated paralysis, suggesting that CD8+ T cells induce neurological disease independent of TCR signaling. Furthermore, spleen and brain CD8+ T cells exhibited antigen-independent cytotoxicity that correlated with NKG2D expression. Finally, viral infection and inflammation in the brain was necessary but not sufficient to induce neurological damage. We demonstrate that CD8+ T cells mediate virus-induced neuropathology via antigen-independent, NKG2D-mediated cytotoxicity, which may serve as a therapeutic target for treatment of virus-induced neurological disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

