Population genetic structure of Aedes aegypti subspecies in selected geographical locations in Sudan
- PMID: 38316804
- PMCID: PMC10844603
- DOI: 10.1038/s41598-024-52591-6
Population genetic structure of Aedes aegypti subspecies in selected geographical locations in Sudan
Abstract
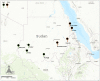
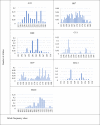
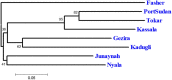
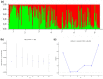
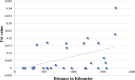
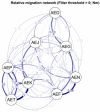
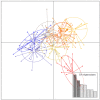
Although knowledge of the composition and genetic diversity of disease vectors is important for their management, this is limiting in many instances. In this study, the population structure and phylogenetic relationship of the two Aedes aegypti subspecies namely Aedes aegypti aegypti (Aaa) and Aedes aegypti formosus (Aaf) in eight geographical areas in Sudan were analyzed using seven microsatellite markers. Hardy-Weinberg Equilibrium (HWE) for the two subspecies revealed that Aaa deviated from HWE among the seven microsatellite loci, while Aaf exhibited departure in five loci and no departure in two loci (A10 and M201). The Factorial Correspondence Analysis (FCA) plots revealed that the Aaa populations from Port Sudan, Tokar, and Kassala clustered together (which is consistent with the unrooted phylogenetic tree), Aaf from Fasher and Nyala populations clustered together, and Gezira, Kadugli, and Junaynah populations also clustered together. The Bayesian cluster analysis structured the populations into two groups suggesting two genetically distinct groups (subspecies). Isolation by distance test revealed a moderate to strong significant correlation between geographical distance and genetic variations (p = 0.003, r = 0.391). The migration network created using divMigrate demonstrated that migration and gene exchange between subspecies populations appear to occur based on their geographical proximity. The genetic structure of the Ae. aegypti subspecies population and the gene flow among them, which may be interpreted as the mosquito vector's capacity for dispersal, were revealed in this study. These findings will help in the improvement of dengue epidemiology research including information on the identity of the target vector/subspecies and the arboviruses vector surveillance program.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

