Transcriptomic analysis reveals the molecular basis of photoperiod-regulated sex differentiation in tropical pumpkins (Cucurbita moschata Duch.)
- PMID: 38317069
- PMCID: PMC10845594
- DOI: 10.1186/s12870-024-04777-3
Transcriptomic analysis reveals the molecular basis of photoperiod-regulated sex differentiation in tropical pumpkins (Cucurbita moschata Duch.)
Abstract
Background: Photoperiod, or the length of the day, has a significant impact on the flowering and sex differentiation of photoperiod-sensitive crops. The "miben" pumpkin (the main type of Cucurbita moschata Duch.) is well-known for its high yield and strong disease resistance. However, its cultivation has been limited due to its sensitivity to photoperiod. This sensitivity imposes challenges on its widespread cultivation and may result in suboptimal yields in regions with specific daylength conditions. As a consequence, efforts are being made to explore potential strategies or breeding techniques to enhance its adaptability to a broader range of photoperiods, thus unlocking its full cultivation potential and further promoting its valuable traits in agriculture.
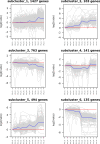
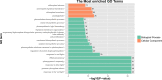
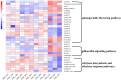
Results: This study aimed to identify photoperiod-insensitive germplasm exhibiting no difference in sex differentiation under different day-length conditions. The investigation involved a phenotypic analysis of photoperiod-sensitive (PPS) and photoperiod-insensitive (PPIS) pumpkin materials exposed to different day lengths, including long days (LDs) and short days (SDs). The results revealed that female flower differentiation was significantly inhibited in PPS_LD, while no differences were observed in the other three groups (PPS_SD, PPIS_LD, and PPIS_SD). Transcriptome analysis was carried out for these four groups to explore the main-effect genes of sex differentiation responsive to photoperiod. The main-effect gene subclusters were identified based on the principal component and hierarchical cluster analyses. Further, functional annotations and enrichment analysis revealed significant upregulation of photoreceptors (CmCRY1, F-box/kelch-repeat protein), circadian rhythm-related genes (CmGI, CmPRR9, etc.), and CONSTANS (CO) in PPS_LD. Conversely, a significant downregulation was observed in most Nuclear Factor Y (NF-Y) transcription factors. Regarding the gibberellic acid (GA) signal transduction pathway, positive regulators of GA signaling (CmSCL3, CmSCL13, and so forth) displayed higher expression levels, while the negative regulators of GA signaling, CmGAI, exhibited lower expression levels in PPS_LD. Notably, this effect was not observed in the synthetic pathway genes. Furthermore, genes associated with ethylene synthesis and signal transduction (CmACO3, CmACO1, CmERF118, CmERF118-like1,2, CmWIN1-like, and CmRAP2-7-like) showed significant downregulation.
Conclusions: This study offered a crucial theoretical and genetic basis for understanding how photoperiod influences the mechanism of female flower differentiation in pumpkins.
Keywords: Cucurbita moschata; Ethylene biosynthetic and ethylene response pathways; Gibberellin signaling pathway; Photoperiod; Photoperiod-mediated flowering processes; Sex differentiation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Gibberellic Acid Signaling Is Required to Induce Flowering of Chrysanthemums Grown under Both Short and Long Days.Int J Mol Sci. 2017 Jun 12;18(6):1259. doi: 10.3390/ijms18061259. Int J Mol Sci. 2017. PMID: 28604637 Free PMC article.
-
Morphological, Transcriptomic and Hormonal Characterization of Trimonoecious and Subandroecious Pumpkin (Cucurbita maxima) Suggests Important Roles of Ethylene in Sex Expression.Int J Mol Sci. 2019 Jun 28;20(13):3185. doi: 10.3390/ijms20133185. Int J Mol Sci. 2019. PMID: 31261811 Free PMC article.
-
Transcriptome profiling of pumpkin (Cucurbita moschata Duch.) leaves infected with powdery mildew.PLoS One. 2018 Jan 10;13(1):e0190175. doi: 10.1371/journal.pone.0190175. eCollection 2018. PLoS One. 2018. PMID: 29320569 Free PMC article.
-
Metabolomics, phytohormone and transcriptomics strategies to reveal the mechanism of barley heading date regulation to responds different photoperiod.BMC Genomics. 2024 Sep 19;25(1):879. doi: 10.1186/s12864-024-10788-z. BMC Genomics. 2024. PMID: 39300396 Free PMC article.
-
Flowering time regulation: photoperiod- and temperature-sensing in leaves.Trends Plant Sci. 2013 Oct;18(10):575-83. doi: 10.1016/j.tplants.2013.05.003. Epub 2013 Jun 18. Trends Plant Sci. 2013. PMID: 23790253 Free PMC article. Review.
Cited by
-
Fine mapping and identification of CqMYB62 as the subgynoecy gene in chieh-qua (Benincasa hispida Cogn. var. Chieh-qua How).Theor Appl Genet. 2025 Apr 9;138(5):96. doi: 10.1007/s00122-025-04872-5. Theor Appl Genet. 2025. PMID: 40204945
-
Salt Stress Leads to Morphological and Transcriptional Changes in Roots of Pumpkins (Cucurbita spp.).Plants (Basel). 2025 May 30;14(11):1674. doi: 10.3390/plants14111674. Plants (Basel). 2025. PMID: 40508347 Free PMC article.
-
Transcriptomic analysis revealed that short-day treatment of seedlings promotes flowering in maize (Zea mays L.).Genes Genomics. 2025 Jul;47(7):855-866. doi: 10.1007/s13258-025-01640-z. Epub 2025 Apr 7. Genes Genomics. 2025. PMID: 40193047
References
-
- Cantliffe DJ. Alteration of sex expression in cucumber due to changes in temperature, light intensity, and photoperiod. J Amer Soc Hort Sci. 1981;106(2):133–6. doi: 10.21273/JASHS.106.2.133. - DOI
-
- Ito H, Saito T. Factors responsible for the sex expression of Japanese cucumber.(IX) effects of nitrogen application and watering under the controlled day length and night temperature in the nursery bed. J Japan Soc Hort Sci. 1958;27(1):11–20. doi: 10.2503/jjshs.27.11. - DOI
-
- Yamasaki S, Fujii N, Takahashi H. Photoperiodic regulation of CS-ACS2, CS-ACS4 and CS-ERS gene expression contributes to the femaleness of cucumber flowers through diurnal ethylene production under short-day conditions. Plant Cell Environ. 2003;26(4):537–46. doi: 10.1046/j.1365-3040.2003.00984.x. - DOI
-
- Krishnamoorthy H, Sandooja J. Effect of ethrel and GA3 on growth, flowering and sex expression of Cucurbita pepo L. Haryana J Hort Sci 1981.
-
- Ma BM, Ms I, Zh S. Altered sex expression by plant growth regulators: an overview in medicinal vegetable bitter gourd (Momordica charantia L) J Med Plants Res. 2014;8(8):361–7. doi: 10.5897/JMPR10.032. - DOI
MeSH terms
Substances
Grants and funding
- R2023PY-JG005/the Special Fund for Scientific Innovation Strategy- Construction of High-Level Academy of Agricultural Sciences
- 202103TD/the Agricultural Competitive Industry Discipline Team Building Project of Guangdong Academy of Agricultural Sciences
- 32302575/the National Natural Science Foundation of China
- 2022A1515110817/the Guangdong Basic and Applied Basic Research Foundation
- 2020B020220003/the Key Realm R&D Program of Guangdong Province
LinkOut - more resources
Full Text Sources
Research Materials

