Platelet-derived growth factor signaling in pericytes promotes hypothalamic inflammation and obesity
- PMID: 38317079
- PMCID: PMC10845801
- DOI: 10.1186/s10020-024-00793-z
Platelet-derived growth factor signaling in pericytes promotes hypothalamic inflammation and obesity
Abstract
Background: Pericytes are a vital component of the blood-brain barrier, and their involvement in acute inflammation was recently suggested. However, it remains unclear whether pericytes contribute to hypothalamic chronic inflammation and energy metabolism in obesity. The present study investigated the impact of pericytes on the pathophysiology of obesity by focusing on platelet-derived growth factor (PDGF) signaling, which regulates pericyte functions.
Methods: Tamoxifen-inducible systemic conditional PDGF receptor β knockout mice (Pdgfrb∆SYS-KO) and Calcium/calmodulin-dependent protein kinase type IIa (CaMKIIa)-positive neuron-specific PDGF receptor β knockout mice (Pdgfrb∆CaMKII-KO) were fed a high-fat diet, and metabolic phenotypes before and 3 to 4 weeks after dietary loading were examined. Intracellular energy metabolism and relevant signal transduction in lipopolysaccharide- and/or platelet-derived growth factor-BB (PDGF-BB)-stimulated human brain pericytes (HBPCs) were assessed by the Seahorse XFe24 Analyzer and Western blotting. The pericyte secretome in conditioned medium from HBPCs was studied using cytokine array kit, and its impact on polarization was examined in bone marrow-derived macrophages (BMDMs), which are microglia-like cells.
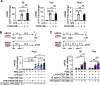
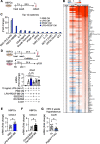
Results: Energy consumption increased and body weight gain decreased after high-fat diet loading in Pdgfrb∆SYS-KO mice. Cellular oncogene fos (cFos) expression increased in proopiomelanocortin (POMC) neurons, whereas microglial numbers and inflammatory gene expression decreased in the hypothalamus of Pdgfrb∆SYS-KO mice. No significant changes were observed in Pdgfrb∆CaMKII-KO mice. In HBPCs, a co-stimulation with lipopolysaccharide and PDGF-BB shifted intracellular metabolism towards glycolysis, activated mitogen-activated protein kinase (MAPK), and modulated the secretome to the inflammatory phenotype. Consequently, the secretome showed an increase in various proinflammatory chemokines and growth factors including Epithelial-derived neutrophil-activating peptide 78 (C-X-C motif chemokine ligand (CXCL)5), Thymus and activation-regulated chemokine (C-C motif chemokine (CCL)17), Monocyte chemoattractant protein 1 (CCL2), and Growth-regulated oncogene α (CXCL1). Furthermore, conditioned medium from HBPCs stimulated the inflammatory priming of BMDMs, and this change was abolished by the C-X-C motif chemokine receptor (CXCR) inhibitor. Consistently, mRNA expression of CXCL5 was elevated by lipopolysaccharide and PDGF-BB treatment in HBPCs, and the expression was significantly lower in the hypothalamus of Pdgfrb∆SYS-KO mice than in control Pdgfrbflox/flox mice (FL) following 4 weeks of HFD feeding.
Conclusions: PDGF receptor β signaling in hypothalamic pericytes promotes polarization of macrophages by changing their secretome and contributes to the progression of obesity.
Keywords: Inflammation; Microglia; Obesity; Pericytes; Platelet-derived growth factor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

