The impact of selective HDAC inhibitors on the transcriptome of early mouse embryos
- PMID: 38317092
- PMCID: PMC10840191
- DOI: 10.1186/s12864-024-10029-3
The impact of selective HDAC inhibitors on the transcriptome of early mouse embryos
Abstract
Background: Histone acetylation, which is regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs), plays a crucial role in the control of gene expression. HDAC inhibitors (HDACi) have shown potential in cancer therapy; however, the specific roles of HDACs in early embryos remain unclear. Moreover, although some pan-HDACi have been used to maintain cellular undifferentiated states in early embryos, the specific mechanisms underlying their effects remain unknown. Thus, there remains a significant knowledge gap regarding the application of selective HDACi in early embryos.
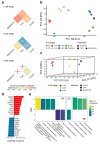
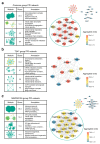
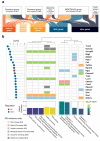
Results: To address this gap, we treated early embryos with two selective HDACi (MGCD0103 and T247). Subsequently, we collected and analyzed their transcriptome data at different developmental stages. Our findings unveiled a significant effect of HDACi treatment during the crucial 2-cell stage of zygotes, leading to a delay in embryonic development after T247 and an arrest at 2-cell stage after MGCD0103 administration. Furthermore, we elucidated the regulatory targets underlying this arrested embryonic development, which pinpointed the G2/M phase as the potential period of embryonic development arrest caused by MGCD0103. Moreover, our investigation provided a comprehensive profile of the biological processes that are affected by HDACi, with their main effects being predominantly localized in four aspects of zygotic gene activation (ZGA): RNA splicing, cell cycle regulation, autophagy, and transcription factor regulation. By exploring the transcriptional regulation and epigenetic features of the genes affected by HDACi, we made inferences regarding the potential main pathways via which HDACs affect gene expression in early embryos. Notably, Hdac7 exhibited a distinct response, highlighting its potential as a key player in early embryonic development.
Conclusions: Our study conducted a comprehensive analysis of the effects of HDACi on early embryonic development at the transcriptional level. The results demonstrated that HDACi significantly affected ZGA in embryos, elucidated the distinct actions of various selective HDACi, and identified specific biological pathways and mechanisms via which these inhibitors modulated early embryonic development.
Keywords: Early embryos; Epigenetic reprogramming; HDAC inhibitor; MGCD0103; T247; Transcriptome; Zygotic gene activation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Effect of histone acetylation modification with MGCD0103, a histone deacetylase inhibitor, on nuclear reprogramming and the developmental competence of porcine somatic cell nuclear transfer embryos.Theriogenology. 2017 Jan 1;87:298-305. doi: 10.1016/j.theriogenology.2016.09.011. Epub 2016 Sep 13. Theriogenology. 2017. PMID: 27742403
-
Histone deacetylase inhibitors modulate the transcriptional regulation of guanylyl cyclase/natriuretic peptide receptor-a gene: interactive roles of modified histones, histone acetyltransferase, p300, AND Sp1.J Biol Chem. 2014 Mar 7;289(10):6991-7002. doi: 10.1074/jbc.M113.511444. Epub 2014 Jan 22. J Biol Chem. 2014. PMID: 24451378 Free PMC article.
-
Histone deacetylase (HDAC) activity is critical for embryonic kidney gene expression, growth, and differentiation.J Biol Chem. 2011 Sep 16;286(37):32775-89. doi: 10.1074/jbc.M111.248278. Epub 2011 Jul 21. J Biol Chem. 2011. PMID: 21778236 Free PMC article.
-
Epigenetic Targeting of Autophagy via HDAC Inhibition in Tumor Cells: Role of p53.Int J Mol Sci. 2018 Dec 8;19(12):3952. doi: 10.3390/ijms19123952. Int J Mol Sci. 2018. PMID: 30544838 Free PMC article. Review.
-
Mocetinostat (MGCD0103): a review of an isotype-specific histone deacetylase inhibitor.Expert Opin Investig Drugs. 2011 Jun;20(6):823-9. doi: 10.1517/13543784.2011.577737. Epub 2011 May 10. Expert Opin Investig Drugs. 2011. PMID: 21554162 Free PMC article. Review.
Cited by
-
A comprehensive review of histone modifications during mammalian oogenesis and early embryo development.Histochem Cell Biol. 2025 Jun 28;163(1):70. doi: 10.1007/s00418-025-02398-x. Histochem Cell Biol. 2025. PMID: 40580240 Free PMC article. Review.
-
Endocrine-disrupting chemicals (EDCs) and epigenetic regulation in embryonic development: Mechanisms, impacts, and emerging trends.Toxicol Rep. 2024 Dec 27;14:101885. doi: 10.1016/j.toxrep.2024.101885. eCollection 2025 Jun. Toxicol Rep. 2024. PMID: 40612660 Free PMC article. Review.
-
RNA polymerase II coordinates histone deacetylation at active promoters.Sci Adv. 2025 Feb 7;11(6):eadt3037. doi: 10.1126/sciadv.adt3037. Epub 2025 Feb 5. Sci Adv. 2025. PMID: 39908363 Free PMC article.
-
RNA Polymerase II coordinates histone deacetylation at active promoters.bioRxiv [Preprint]. 2024 Sep 17:2024.09.17.613553. doi: 10.1101/2024.09.17.613553. bioRxiv. 2024. Update in: Sci Adv. 2025 Feb 07;11(6):eadt3037. doi: 10.1126/sciadv.adt3037. PMID: 39345547 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

