Targeting of H19/cell adhesion molecules circuitry by GSK-J4 epidrug inhibits metastatic progression in prostate cancer
- PMID: 38317193
- PMCID: PMC10845766
- DOI: 10.1186/s12935-024-03231-6
Targeting of H19/cell adhesion molecules circuitry by GSK-J4 epidrug inhibits metastatic progression in prostate cancer
Abstract
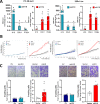
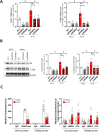
Background: About 30% of Prostate cancer (PCa) patients progress to metastatic PCa that remains largely incurable. This evidence underlines the need for the development of innovative therapies. In this direction, the potential research focus might be on long non-coding RNAs (lncRNAs) like H19, which serve critical biological functions and show significant dysregulation in cancer. Previously, we showed a transcriptional down-regulation of H19 under combined pro-tumoral estrogen and hypoxia treatment in PCa cells that, in turn, induced both E-cadherin and β4 integrin expression. H19, indeed, acts as transcriptional repressor of cell adhesion molecules affecting the PCa metastatic properties. Here, we investigated the role of H19/cell adhesion molecules circuitry on in vivo PCa experimental tumor growth and metastatic dissemination models.
Methods: H19 was silenced in luciferase-positive PC-3 and 22Rv1 cells and in vitro effect was evaluated by gene expression, proliferation and invasion assays before and after treatment with the histone lysine demethylase inhibitor, GSK-J4. In vivo tumor growth and metastasis dissemination, in the presence or absence of GSK-J4, were analyzed in two models of human tumor in immunodeficient mice by in vivo bioluminescent imaging and immunohistochemistry (IHC) on explanted tissues. Organotypic Slice Cultures (OSCs) from fresh PCa-explant were used as ex vivo model to test GSK-J4 effects.
Results: H19 silencing in both PC-3 and 22Rv1 cells increased: i) E-cadherin and β4 integrin expression as well as proliferation and invasion, ii) in vivo tumor growth, and iii) metastasis formation at bone, lung, and liver. Of note, treatment with GSK-J4 reduced lesions. In parallel, GSK-J4 efficiently induced cell death in PCa-derived OSCs.
Conclusions: Our findings underscore the potential of the H19/cell adhesion molecules circuitry as a targeted approach in PCa treatment. Modulating this interaction has proven effective in inhibiting tumor growth and metastasis, presenting a logical foundation for targeted therapy.
Keywords: Lysine demethylase; Metastasis; Preclinical models; lncRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

