The novel phosphatase NUDT5 is a critical regulator of triple-negative breast cancer growth
- PMID: 38317231
- PMCID: PMC10845800
- DOI: 10.1186/s13058-024-01778-w
The novel phosphatase NUDT5 is a critical regulator of triple-negative breast cancer growth
Erratum in
-
Correction: The novel phosphatase NUDT5 is a critical regulator of triple-negative breast cancer growth.Breast Cancer Res. 2024 Mar 26;26(1):53. doi: 10.1186/s13058-024-01814-9. Breast Cancer Res. 2024. PMID: 38532428 Free PMC article. No abstract available.
Abstract
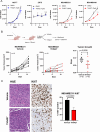
Background: The most aggressive form of breast cancer is triple-negative breast cancer (TNBC), which lacks expression of the estrogen receptor (ER) and progesterone receptor (PR), and does not have overexpression of the human epidermal growth factor receptor 2 (HER2). Treatment options for women with TNBC tumors are limited, unlike those with ER-positive tumors that can be treated with hormone therapy, or those with HER2-positive tumors that can be treated with anti-HER2 therapy. Therefore, we have sought to identify novel targeted therapies for TNBC. In this study, we investigated the potential of a novel phosphatase, NUDT5, as a potential therapeutic target for TNBC.
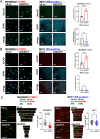
Methods: The mRNA expression levels of NUDT5 in breast cancers were investigated using TCGA and METABRIC (Curtis) datasets. NUDT5 ablation was achieved through siRNA targeting and NUDT5 inhibition with the small molecule inhibitor TH5427. Xenograft TNBC animal models were employed to assess the effect of NUDT5 inhibition on in vivo tumor growth. Proliferation, death, and DNA replication assays were conducted to investigate the cellular biological effects of NUDT5 loss or inhibition. The accumulation of 8-oxo-guanine (8-oxoG) and the induction of γH2AX after NUDT5 loss was determined by immunofluorescence staining. The impact of NUDT5 loss on replication fork was assessed by measuring DNA fiber length.
Results: In this study, we demonstrated the significant role of an overexpressed phosphatase, NUDT5, in regulating oxidative DNA damage in TNBCs. Our findings indicate that loss of NUDT5 results in suppressed growth of TNBC both in vitro and in vivo. This growth inhibition is not attributed to cell death, but rather to the suppression of proliferation. The loss or inhibition of NUDT5 led to an increase in the oxidative DNA lesion 8-oxoG, and triggered the DNA damage response in the nucleus. The interference with DNA replication ultimately inhibited proliferation.
Conclusions: NUDT5 plays a crucial role in preventing oxidative DNA damage in TNBC cells. The loss or inhibition of NUDT5 significantly suppresses the growth of TNBCs. These biological and mechanistic studies provide the groundwork for future research and the potential development of NUDT5 inhibitors as a promising therapeutic approach for TNBC patients.
Keywords: Oxidative stress; Phosphatase; Triple-negative breast cancer.
© 2024. The Author(s).
Conflict of interest statement
P.H.B served as a Scientific Advisory Board Member for the Susan G. Komen for the Cure Foundation (until 2017), and P.H.B is a holder of GeneTex stock (less than 1% of the total company stock); neither of these relate to this publication. All remaining authors declare no actual, potential, or perceived conflict of interest that would prejudice the impartiality of this article.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

