Cognitive, functional, and neuropsychiatric correlates of regional tau pathology in autopsy-confirmed chronic traumatic encephalopathy
- PMID: 38317248
- PMCID: PMC10845638
- DOI: 10.1186/s13024-023-00697-2
Cognitive, functional, and neuropsychiatric correlates of regional tau pathology in autopsy-confirmed chronic traumatic encephalopathy
Abstract
Background: Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease characterized by hyperphosphorylated tau (p-tau) accumulation. The clinical features associated with CTE pathology are unclear. In brain donors with autopsy-confirmed CTE, we investigated the association of CTE p-tau pathology density and location with cognitive, functional, and neuropsychiatric symptoms.
Methods: In 364 brain donors with autopsy confirmed CTE, semi-quantitative p-tau severity (range: 0-3) was assessed in 10 cortical and subcortical regions. We summed ratings across regions to form a p-tau severity global composite (range: 0-30). Informants completed standardized scales of cognition (Cognitive Difficulties Scale, CDS; BRIEF-A Metacognition Index, MI), activities of daily living (Functional Activities Questionnaire), neurobehavioral dysregulation (BRIEF-A Behavioral Regulation Index, BRI; Barratt Impulsiveness Scale, BIS-11), aggression (Brown-Goodwin Aggression Scale), depression (Geriatric Depression Scale-15, GDS-15), and apathy (Apathy Evaluation Scale, AES). Ordinary least squares regression models examined associations between global and regional p-tau severity (separate models for each region) with each clinical scale, adjusting for age at death, racial identity, education level, and history of hypertension, obstructive sleep apnea, and substance use treatment. Ridge regression models that incorporated p-tau severity across all regions in the same model assessed which regions showed independent effects.
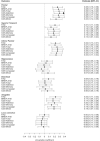
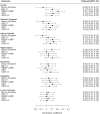
Results: The sample was predominantly American football players (333; 91.2%); 140 (38.5%) had low CTE and 224 (61.5%) had high CTE. Global p-tau severity was associated with higher (i.e., worse) scores on the cognitive and functional scales: MI ([Formula: see text] standardized = 0.02, 95%CI = 0.01-0.04), CDS ([Formula: see text] standardized = 0.02, 95%CI = 0.01-0.04), and FAQ ([Formula: see text] standardized = 0.03, 95%CI = 0.01-0.04). After false-discovery rate correction, p-tau severity in the frontal, inferior parietal, and superior temporal cortex, and the amygdala was associated with higher CDS ([Formula: see text] sstandardized = 0.17-0.29, ps < 0.01) and FAQ ([Formula: see text] sstandardized = 0.21-0.26, ps < 0.01); frontal and inferior parietal cortex was associated with higher MI ([Formula: see text] sstandardized = 0.21-0.29, ps < 0.05); frontal cortex was associated with higher BRI ([Formula: see text] standardized = 0.21, p < 0.01). Regions with effects independent of other regions included frontal cortex (CDS, MI, FAQ, BRI), inferior parietal cortex (CDS) and amygdala (FAQ). P-tau explained 13-49% of variance in cognitive and functional scales and 6-14% of variance in neuropsychiatric scales.
Conclusion: Accumulation of p-tau aggregates, especially in the frontal cortex, are associated with cognitive, functional, and certain neurobehavioral symptoms in CTE.
Keywords: Activities of daily living; Amygdala; Behavioral dysregulation; Chronic traumatic encephalopathy; Clinicopathological correlation; Cognition; Frontal cortex; Tau; Temporal cortex; Traumatic brain injury.
© 2023. The Author(s).
Conflict of interest statement
Dr. Goldstein is a paid consultant to Johnson & Johnson, Janssen Research & Development LLC, and Rebiscan Inc and has received funding from the WWE (World Wrestling Entertainment) and Ivivi Health Sciences.
Dr. Nowinski is the Co-Founder and Chief Executive Officer of the Concussion Legacy Foundation, and serves as an advisor for Oxeia Biopharmaceuticals and PreCon Health, Inc.
Dr. Stern is a paid consultant to Biogen, is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association, receives royalties for published neuropsychological tests from Psychological Assessment Resources Inc, is a member of the Board of Directors of King-Devick Technologies, and reported grants from the National Institutes of Health during the conduct of the study.
Dr. Cantu is a paid consultant to the National Football League Head Neck and Spine Committee, a vice president and chair of the scientific advisory committee of the National Operating Committee on Standards for Athletic Equipment, and a consultant to the Concussion Legacy Foundation; he also receives royalties from Houghton Mifflin Harcourt and compensation for expert legal opinion to the National Collegiate Athletic Association and National Hockey League and is a member of the Mackey-White Committee of the National Football League Players Association.
Dr. McKee is a member of the Mackey-White Committee of the National Football League Players Association and reports receiving grants from the National Institutes of Health and Department of Veteran Affairs and other funding from Buoniconti Foundation during the conduct of the study.
Dr. Alosco reported grants from National Institutes of Health during the conduct of the study.
Dr. Katz reported grants from Boston University School of Medicine Department of Neurology during the conduct of the study, receives royalties from Springer/Demos Publishing for a text book on brain injury, serves as an expert witness in legal cases involving brain injury and concussion; receives a stipend from Encompass Health as program medical director for brain injury and chair of the annual Neurorehabilitation conference; has received honoraria for a keynote address for the HealthSouth annual Medical Directors meeting.
Dr. Mez reported grants from the National Institutes of Health, Department of Defense, Alzheimer’s Association, and Concussion Legacy Foundation during the conduct of the study.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R21HD089088/AG/NIA NIH HHS/United States
- RF1 NS115268/NS/NINDS NIH HHS/United States
- F32NS096803/AG/NIA NIH HHS/United States
- U54NS115266/NS/NINDS NIH HHS/United States
- R01NS078337/NS/NINDS NIH HHS/United States
- 1UL1TR001430/TR/NCATS NIH HHS/United States
- U54 NS115266/NS/NINDS NIH HHS/United States
- R01AG061028/AG/NIA NIH HHS/United States
- I01 CX001038/CX/CSRD VA/United States
- RF1 AG062348/AG/NIA NIH HHS/United States
- K23AG046377/AG/NIA NIH HHS/United States
- F32AG056098/AG/NIA NIH HHS/United States
- U01NS086659/NS/NINDS NIH HHS/United States
- R01 AG062348/AG/NIA NIH HHS/United States
- U01NS093334/NS/NINDS NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- R01AG057902/AG/NIA NIH HHS/United States
- P30 AG072978/AG/NIA NIH HHS/United States
- R01AG062348/AG/NIA NIH HHS/United States
- NIRG-15-362697/ALZ/Alzheimer's Association/United States
- K23 NS102399/NS/NINDS NIH HHS/United States
- R01AG1649/AG/NIA NIH HHS/United States
- R56NS078337/NS/NINDS NIH HHS/United States
- K23NS102399/NS/NINDS NIH HHS/United States
- I01 CX001135/CX/CSRD VA/United States
- NIRG-305779/ALZ/Alzheimer's Association/United States
- RF1 NS095252/NS/NINDS NIH HHS/United States
- RF1 AG057902/AG/NIA NIH HHS/United States
- F32 AG056098/AG/NIA NIH HHS/United States
- P30AG13846; supplement 0572063345/AG/NIA NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- W81XWH‑13–2‑0095/Department of Defense
- W81XWH‑13–2‑0064/Department of Defense
- W81XWH1810580/Department of Defense
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

