Relative deficiency in interferon-γ-secreting CD4+ T cells is strongly associated with poorer COVID-19 vaccination responses in older adults
- PMID: 38317404
- PMCID: PMC11019126
- DOI: 10.1111/acel.14099
Relative deficiency in interferon-γ-secreting CD4+ T cells is strongly associated with poorer COVID-19 vaccination responses in older adults
Abstract
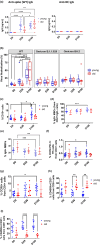
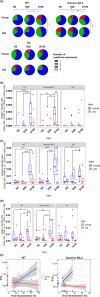
Although the two-dose mRNA vaccination regime provides protection against SARS-CoV-2, older adults have been shown to exhibit poorer vaccination responses. In addition, the role of vaccine-induced T-cell responses is not well characterised. We aim to assess the impact of age on immune responses after two doses of the BNT162b2 mRNA vaccine, focussing on antigen-specific T-cells. A prospective 3-month study was conducted on 15 young (median age 31 years, interquartile range (IQR) 25-35 years) and 14 older adults (median age 72 years, IQR 70-73 years). We assessed functional, neutralising antibody responses against SARS-CoV-2 variants using ACE-2 inhibition assays, and changes in B and T-cell subsets by high-dimensional flow cytometry. Antigen-specific T-cell responses were also quantified by intracellular cytokine staining and flow cytometry. Older adults had attenuated T-helper (Th) response to vaccination, which was associated with weaker antibody responses and decreased SARS-CoV-2 neutralisation. Antigen-specific interferon-γ (IFNγ)-secreting CD4+ T-cells to wild-type and Omicron antigens increased in young adults, which was strongly positively correlated with their neutralising antibody responses. Conversely, this relationship was negative in older adults. Hence, older adults' relative IFNγ-secreting CD4+ T cell deficiency might explain their poorer COVID-19 vaccination responses. Further exploration into the aetiology is needed and would be integral in developing novel vaccination strategies and improving infection outcomes in older adults.
Keywords: COVID‐19 mRNA vaccine; IFN‐γ; antigen‐specific T‐cell response; immunosenescence; older adults.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
C.W.M.O. reports speaker fees from Qiagen travel support from MSD and grant from Institut Merieux outside this work.
Figures
Similar articles
-
The impact of vaccine booster doses on specific B- and T-lymphocyte dynamics in Thai healthcare personnel following COVID-19 vaccination.Sci Rep. 2025 Jul 16;15(1):25713. doi: 10.1038/s41598-025-10400-8. Sci Rep. 2025. PMID: 40670524 Free PMC article.
-
Safety and immunogenicity of a modified mRNA-lipid nanoparticle vaccine candidate against COVID-19: Results from a phase 1, dose-escalation study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2408863. doi: 10.1080/21645515.2024.2408863. Epub 2024 Oct 18. Hum Vaccin Immunother. 2024. PMID: 39422261 Free PMC article. Clinical Trial.
-
Effective cellular and neutralizing immunity against SARS-CoV-2 after mRNA booster vaccination is associated with pDC and B cell activation.Front Immunol. 2025 May 12;16:1580448. doi: 10.3389/fimmu.2025.1580448. eCollection 2025. Front Immunol. 2025. PMID: 40421016 Free PMC article.
-
Detection of SARS-CoV-2-Specific Antibodies in Human Breast Milk and Their Neutralizing Capacity after COVID-19 Vaccination: A Systematic Review.Int J Mol Sci. 2023 Feb 3;24(3):2957. doi: 10.3390/ijms24032957. Int J Mol Sci. 2023. PMID: 36769279 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article.
Cited by
-
Immune Durability and Breakthrough Infections 15 Months After SARS-CoV-2 Boosters in People over 65: The IMMERSION Study.Vaccines (Basel). 2025 Jul 9;13(7):738. doi: 10.3390/vaccines13070738. Vaccines (Basel). 2025. PMID: 40733715 Free PMC article.
References
-
- Briceño, O. , Lissina, A. , Wanke, K. , Afonso, G. , Braun, A. , Ragon, K. , Miquel, T. , Gostick, E. , Papagno, L. , Stiasny, K. , Price, D. A. , Mallone, R. , Sauce, D. , Karrer, U. , & Appay, V. (2016). Reduced naïve CD8(+) T‐cell priming efficacy in elderly adults. Aging Cell, 15(1), 14–21. 10.1111/acel.12384 - DOI - PMC - PubMed
-
- Collier, D. A. , Ferreira, I. A. T. M. , Kotagiri, P. , Datir, R. P. , Lim, E. Y. , Touizer, E. , Meng, B. , Abdullahi, A. , The CITIID‐NIHR BioResource COVID‐19 Collaboration , Principal Investigators , Baker, S. , Dougan, G. , Hess, C. , Kingston, N. , Lehner, P. J. , Lyons, P. A. , Matheson, N. J. , Owehand, W. H. , Saunders, C. , … Gupta, R. K. (2021). Age‐related immune response heterogeneity to SARS‐CoV‐2 vaccine BNT162b2. Nature, 596(7872), 417–422. 10.1038/s41586-021-03739-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- National University of Singapore Infectious Diseases Translational Research Programme Seed Fund
- SUJ#022388-00001/Ministry of Education Singapore
- #75F40120C00085/U.S. Food and Drug Administration
- NUHSRO/2021/032/NUSMedCovid//National University Health System
- COVID19RF-0011; COVID19RF/National Medical Research Council
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

