C5L2 CRISPR KO enhances dental pulp stem cell-mediated dentinogenesis via TrkB under TNFα-induced inflammation
- PMID: 38318114
- PMCID: PMC10839780
- DOI: 10.3389/fcell.2024.1338419
C5L2 CRISPR KO enhances dental pulp stem cell-mediated dentinogenesis via TrkB under TNFα-induced inflammation
Abstract
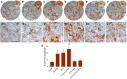
Background and Objectives: Dental caries is one of the most common human pathological conditions resulting from the invasion of bacteria into the dentin. Current treatment options are limited. In many cases, endodontic therapy leads to permanent pulp tissue loss. Dentin-pulp complex regeneration involves dental pulp stem cells (DPSCs) that differentiate into odontoblast-like cells under an inflammatory context. However, limited information is available on how DPSC differentiation processes are affected under inflammatory environments. We identified the crucial role of complement C5a and its receptor C5aR in the inflammation-induced odontoblastic DPSC differentiation. Methodology: Here, we further investigated the role of a second and controversial C5a receptor, C5L2, in this process and explored the underlying mechanism. Human DPSCs were examined during 7-, 10-, and 14-day odontogenic differentiation treated with TNFα, C5L2 CRISPR, and tyrosine receptor kinase B (TrkB) antagonist [cyclotraxin-B (CTX-B)]. Results: Our data demonstrate that C5L2 CRISPR knockout (KO) enhances mineralization in TNFα-stimulated differentiating DPSCs. We further confirmed that C5L2 CRISPR KO significantly enhances dentin sialophosphoprotein (DSPP) and dentin matrix protein-1 (DMP-1) expression after 14-day odontoblastic DPSC differentiation, and treatment with CTX-B abolished the TNFα/C5L2 CRISPR KO-induced DSPP and DMP-1 increase, suggesting TrkB's critical role in this process. Conclusion and Key applications: Our data suggest a regulatory role of C5L2 and TrkB in the TNFα-induced odontogenic DPSC differentiation. This study may provide a useful tool to understand the mechanisms of the role of inflammation in dentinogenesis that is required for successful DPSC engineering strategies.
Keywords: C5L2; DPSC; TrkB; complement C5a; dentinogenesis; inflammation.
Copyright © 2024 Irfan, Marzban and Chung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

