Long-term safety, tolerability, and efficacy of efgartigimod (ADAPT+): interim results from a phase 3 open-label extension study in participants with generalized myasthenia gravis
- PMID: 38318236
- PMCID: PMC10842202
- DOI: 10.3389/fneur.2023.1284444
Long-term safety, tolerability, and efficacy of efgartigimod (ADAPT+): interim results from a phase 3 open-label extension study in participants with generalized myasthenia gravis
Abstract
Objective: ADAPT+ assessed the long-term safety, tolerability, and efficacy of efgartigimod in adult participants with generalized myasthenia gravis (gMG).
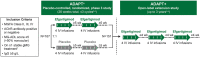
Methods: ADAPT+ was an open-label, single-arm, multicenter, up to 3-year extension of the pivotal phase 3 ADAPT study. Efgartigimod was administered in treatment cycles of 4 intravenous infusions (one 10 mg/kg infusion per week). Initiation of subsequent treatment cycles was individualized based on clinical evaluation. Safety endpoints included incidence and severity of adverse events. Efficacy endpoints assessed disease severity using Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores.
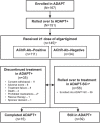
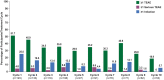
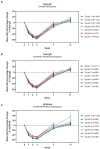
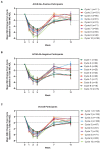
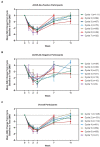
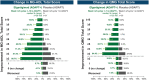
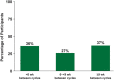
Results: As of January 2022, 151 participants had rolled over to ADAPT+ and 145 had received ≥1 dose of efgartigimod, of whom, 111 (76.6%) were AChR-Ab+ and 34 (23.4%) were AChR-Ab-. Mean study duration (treatment plus follow-up) was 548 days, and participants received up to 17 treatment cycles, corresponding to 217.6 participant-years of exposure. In the overall population, 123 (84.8%) participants reported ≥1 treatment-emergent adverse event; most frequent were headache (36 [24.8%]), COVID-19 (22 [15.2%]), and nasopharyngitis (20 [13.8%]). Clinically meaningful improvement (CMI) in mean MG-ADL and QMG scores was seen as early as 1 week following the first infusion across multiple cycles in AChR-Ab+ and AChR-Ab- participants. Maximal MG-ADL and QMG improvements aligned with onset and magnitude of total IgG and AChR-Ab reductions. For AChR-Ab+ participants at any time point in each of the first 10 treatment cycles, more than 90% had a maximum reduction of ≥2 points (CMI) in MG-ADL total score; across the 7 cycles in which QMG was measured, 69.4% to 91.3% of participants demonstrated a maximum reduction of ≥3 points (CMI) in QMG total score. Many participants demonstrated improvements well beyond CMI thresholds. In AChR-Ab+ participants with ≥1 year of combined follow-up between ADAPT and ADAPT+, mean number of annualized cycles was 4.7 per year (median [range] 5.0 [0.5-7.6]).
Conclusion: Results of ADAPT+ corroborate the substantial clinical improvements seen with efgartigimod in ADAPT and support its long-term safety, tolerability, and efficacy, as well as an individualized dosing regimen for treatment of gMG.
Clinical trial registration: https://classic.clinicaltrials.gov/ct2/show/NCT03770403, NCT03770403.
Keywords: FcRn; IgG recycling; antibody fragment; autoantibody reduction; efgartigimod; myasthenia gravis; neonatal Fc receptor; neonatal Fc receptor antagonist.
Copyright © 2024 Howard, Bril, Vu, Karam, Peric, De Bleecker, Murai, Meisel, Beydoun, Pasnoor, Guglietta, Van Hoorick, Steeland, T’joen, Utsugisawa, Verschuuren, Mantegazza, the ADAPT+ Study Group.
Conflict of interest statement
SS, CT’j, and BH are employees of argenx, Ghent, Belgium. AG was an employee of argenx during study execution and at the initiation of this manuscript; he is currently an independent consultant for the biotechnology and pharmaceutical industry. JH has received research support (paid to his institution) from Alexion Pharmaceuticals, argenx, Cartesian Therapeutics, the Centers for Disease Control and Prevention (Atlanta, GA, USA), the Myasthenia Gravis Foundation of America, the Muscular Dystrophy Association, the National Institutes of Health (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), PCORI, Ra Pharmaceuticals (now UCB Biosciences), and Millennium Pharmaceuticals/Takeda Pharmaceuticals; honoraria from AcademicCME, Alexion Pharmaceuticals, argenx, Biologix Pharma, F. Hoffman-LaRoche Ltd., Horizon Therapeutics, Immunovant Inc., Medscape CME, Merck EMB Serono, Novartis Pharmaceuticals, PeerView CME, Ra Pharmaceuticals (now UCB Biosciences), Regeneron Pharmaceuticals, Sanofi US, and Zai Laboratories; and non-financial support from Alexion Pharmaceuticals, argenx BVBA, Ra Pharmaceuticals (now UCB Biosciences), and Toleranzia AB. VB has participated in scientific advisory boards for CSL Behring, Baxalta, Grifols, argenx, Octapharma, Alpha Technologies, Powell Mansfield Inc., Shire, Akcea, UCB, and Alnylam. She has received funding for travel or speaker honoraria from CSL Behring and has consultancies with CSL Behring, Grifols, BioNevia, Octapharma, Powell Mansfield Inc., argenx, Alpha Technologies, Baxalta, Akcea, UCB, Alnylam, and Pfizer. TV served as site principal investigator for MG clinical trials sponsored by Alexion, argenx, Ra/UCB, Horizon/Viela Bio, Janssen/Momenta, Regeneron, and Cartesian Therapeutics and is a consultant for UCB, Alexion, Dianthus, and argenx. CK has participated in scientific advisory boards for CSL Behring, Takeda, argenx, AstraZeneca, Alexion, Alpine, Corino, Ionis, Sanofi, UCB, and Alnylam. He has received research funding from Ionis. JV has been involved in MG research sponsored by the Prinses Beatrix Spierfonds, Health Holland, and consultancies for argenx, Alexion, and NMD Pharma. Reimbursements were received by the LUMC. He is coinventor on patent applications based on MuSK-related research. He is a member of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD). SP has served as site principal investigator for MG clinical trials sponsored by argenx, Ra/UCB, Takeda/Millennium, and Alexion and is a consultant for argenx and Dianthus. JB has served as a paid consultant for or received speaker honoraria from argenx, UCB Pharma, Alexion Pharmaceuticals, Sanofi, and Biogen. HM has served as a paid consultant for Alexion, AstraZeneca Rare Disease, argenx, UCB Pharma, and Roche and has received speaker honoraria from the Japan Blood Products Organization and Chugai Pharmaceutical and research support from the Ministry of Health, Labour and Welfare, Japan. AM has served as advisor, consultant, principal investigator, or speaker and has received research grants (paid to his employer) and honoraria from Alexion, argenx, Axunio, Grifols, Hormosan, Janssen, Merck, Octapharma, and UCB. He serves as chair of the medical advisory board of the German Myasthenia Gravis Society. SB has participated in research studies sponsored by argenx, Sanofi, UCB, Regeneron, Genentech, Janssen, Amylyx, and Healey ALS. He is a paid consultant and speaker for Takeda, Alnylam, Alexion, Grifols, Amylyx, argenx, and CSL. MP has served as a consultant or medical advisor to Terumo BCT, Alexion, CSL Behring, Momenta, Catalyst, UCB, Immunovant, and Janssen. KU has served as a paid consultant for argenx, UCB Pharma, Janssen Pharma, Viela Bio, Chugai Pharma, Merck, and Mitsubishi Tanabe Pharma and has received speaker honoraria from argenx, Alexion Pharmaceuticals, UCB Pharma, and the Japan Blood Products Organization. RM has received funding for travel, meeting attendance, and advisory board participation from Alexion, argenx, Biomarin, Catalyst, Sanofi, Regeneron, and UCB.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

