Deficient tRNA posttranscription modification dysregulated the mitochondrial quality controls and apoptosis
- PMID: 38318358
- PMCID: PMC10838789
- DOI: 10.1016/j.isci.2024.108883
Deficient tRNA posttranscription modification dysregulated the mitochondrial quality controls and apoptosis
Abstract
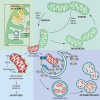
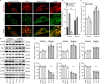
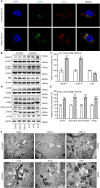
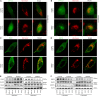
Mitochondria are dynamic organelles in cellular metabolism and physiology. Mitochondrial DNA (mtDNA) mutations are associated with a broad spectrum of clinical abnormalities. However, mechanisms underlying mtDNA mutations regulate intracellular signaling related to the mitochondrial and cellular integrity are less explored. Here, we demonstrated that mt-tRNAMet 4435A>G mutation-induced nucleotide modification deficiency dysregulated the expression of nuclear genes involved in cytosolic proteins involved in oxidative phosphorylation system (OXPHOS) and impaired the assemble and integrity of OXPHOS complexes. These dysfunctions caused mitochondrial dynamic imbalance, thereby increasing fission and decreasing fusion. Excessive fission impaired the process of autophagy including initiation phase, formation, and maturation of autophagosome. Strikingly, the m.4435A>G mutation upregulated the PARKIN dependent mitophagy pathways but downregulated the ubiquitination-independent mitophagy. These alterations promoted intrinsic apoptotic process for the removal of damaged cells. Our findings provide new insights into mechanism underlying deficient tRNA posttranscription modification regulated intracellular signaling related to the mitochondrial and cellular integrity.
Keywords: Cell biology; Molecular physiology; Properties of biomolecules.
© 2024 The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

