Comparison of glycosyl donors: a supramer approach
- PMID: 38318458
- PMCID: PMC10840533
- DOI: 10.3762/bjoc.20.18
Comparison of glycosyl donors: a supramer approach
Abstract
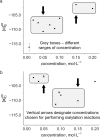
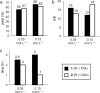
The development of new methods for chemical glycosylation commonly includes comparison of various glycosyl donors. An attempted comparison of chemical properties of two sialic acid-based thioglycoside glycosyl donors, differing only in the substituent at O-9 (trifluoroacetyl vs chloroacetyl), at different concentrations (0.05 and 0.15 mol·L-1) led to mutually excluding conclusions concerning their relative reactivity and selectivity, which prevented us from revealing a possible influence of remote protective groups at O-9 on glycosylation outcome. According to the results of the supramer analysis of the reaction solutions, this issue might be related to the formation of supramers of glycosyl donors differing in structure hence chemical properties. These results seem to imply that comparison of chemical properties of different glycosyl donors may not be as simple and straightforward as it is usually considered.
Keywords: concentration; glycosylation; protecting groups; reactivity; sialic acids; stereoselectivity.
Copyright © 2024, Orlova et al.
Figures

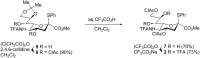
References
LinkOut - more resources
Full Text Sources
