Design of dual peptide-conjugated hydrogels for proliferation and differentiation of human pluripotent stem cells
- PMID: 38318478
- PMCID: PMC10839443
- DOI: 10.1016/j.mtbio.2024.100969
Design of dual peptide-conjugated hydrogels for proliferation and differentiation of human pluripotent stem cells
Abstract
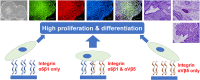
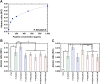
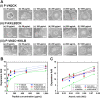
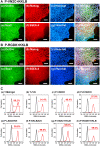
Completely synthetic cell cultivation materials for human pluripotent stem cells (hPSCs) are important for the future clinical use of hPSC-derived cells. Currently, cell culture materials conjugated with extracellular matrix (ECM)-derived peptides are being prepared using only one specific integrin-targeting peptide. We designed dual peptide-conjugated hydrogels, for which each peptide was selected from different ECM sites: the laminin β4 chain and fibronectin or vitronectin, which can target α6β1 and α2β1 or αVβ5. hPSCs cultured on dual peptide-conjugated hydrogels, especially on hydrogels conjugated with peptides obtained from the laminin β4 chain and vitronectin with a low peptide concentration of 200 μg/mL, showed high proliferation ability over the long term and differentiated into cells originating from 3 germ layers in vivo as well as a specific lineage of cardiac cells. The design of grafting peptides was also important, for which a joint segment and positive amino acids were added into the designed peptide. Because of the designed peptides on the hydrogels, only 200 μg/mL peptide solution was sufficient for grafting on the hydrogels, and the hydrogels supported hPSC cultures long-term; in contrast, in previous studies, greater than 1000 μg/mL peptide solution was needed for the grafting of peptides on cell culture materials.
Keywords: Cardiomyocyte; Human pluripotent stem cells; Hydrogel; Integrin; Peptide; Proliferation.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Akon Higuchi reports financial support was provided by 10.13039/501100012166National Key Research and Development Program of China. Akon Higuchi reports financial support was provided by 10.13039/501100001809National Natural Science Foundation of China. Tzu-Cheng Sung reports financial support was provided by 10.13039/501100007194Wenzhou Municipal Science and Technology Bureau. Akon Higuchi reports financial support was provided by 10.13039/501100007194Wenzhou Municipal Science and Technology Bureau. Akon Higuchi reports financial support was provided by 10.13039/501100011912Taipei Veterans General Hospital. Akon Higuchi reports financial support was provided by National Defense Medical Center. Henry Hsin-Chung Lee reports financial support was provided by 10.13039/501100002811Cathay General Hospital. Akon Higuchi reports financial support was provided by National Science and Technology Council. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Designed peptide-grafted hydrogels for human pluripotent stem cell culture and differentiation.J Mater Chem B. 2023 Feb 15;11(7):1434-1444. doi: 10.1039/d2tb02521c. J Mater Chem B. 2023. PMID: 36541288
-
Human embryonic stem cells cultured on hydrogels grafted with extracellular matrix protein-derived peptides with polyethylene glycol joint nanosegments.IET Nanobiotechnol. 2022 Dec;16(9):295-304. doi: 10.1049/nbt2.12091. Epub 2022 Oct 6. IET Nanobiotechnol. 2022. PMID: 36200801 Free PMC article.
-
Poly(vinyl alcohol-co-itaconic acid) hydrogels grafted with several designed peptides for human pluripotent stem cell culture and differentiation into cardiomyocytes.J Mater Chem B. 2021 Sep 29;9(37):7662-7673. doi: 10.1039/d1tb01555a. J Mater Chem B. 2021. PMID: 34586153
-
Xeno-free culture and proliferation of hPSCs on 2D biomaterials.Prog Mol Biol Transl Sci. 2023;199:63-107. doi: 10.1016/bs.pmbts.2023.02.008. Epub 2023 Mar 14. Prog Mol Biol Transl Sci. 2023. PMID: 37678982 Review.
-
Cell-binding peptides on the material surface guide stem cell fate of adhesion, proliferation and differentiation.J Mater Chem B. 2023 Feb 15;11(7):1389-1415. doi: 10.1039/d2tb02601e. J Mater Chem B. 2023. PMID: 36727243 Review.
Cited by
-
Differentiation of human induced pluripotent stem cells into retinal pigment epithelium cells during culture on peptide-grafted hydrogels.Regen Biomater. 2025 Apr 26;12:rbaf035. doi: 10.1093/rb/rbaf035. eCollection 2025. Regen Biomater. 2025. PMID: 40416648 Free PMC article.
-
Biomolecule-functionalized dental implant surfaces: Towards augmenting soft tissue integration.Bioact Mater. 2025 Jul 26;53:540-590. doi: 10.1016/j.bioactmat.2025.07.005. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40755849 Free PMC article. Review.
-
Smart responsive in situ hydrogel systems applied in bone tissue engineering.Front Bioeng Biotechnol. 2024 May 28;12:1389733. doi: 10.3389/fbioe.2024.1389733. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38863497 Free PMC article. Review.
-
Synthetic hydrogel substrate for human induced pluripotent stem cell definitive endoderm differentiation.Biomaterials. 2025 Apr;315:122920. doi: 10.1016/j.biomaterials.2024.122920. Epub 2024 Oct 25. Biomaterials. 2025. PMID: 39504708
References
-
- Golchin A., Chatziparasidou A., Ranjbarvan P., Niknam Z., Ardeshirylajimi A. Embryonic stem cells in clinical trials: current overview of developments and challenges. Adv. Exp. Med. Biol. 2021;1312:19–37. - PubMed
-
- Higuchi A., Kumar S.S., Benelli G., Ling Q.D., Li H.F., Alarfaj A.A., Munusamy M.A., Sung T.C., Chang Y., Murugan K. Biomaterials used in stem cell therapy for spinal cord injury. Prog. Mater. Sci. 2019;103:374–424.
-
- Higuchi A., Kumar S.S., Benelli G., Alarfaj A.A., Munusamy M.A., Umezawa A., Murugan K. Stem cell therapies for reversing vision loss. Trends Biotechnol. 2017;35(11):1102–1117. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

