Fluorescent ratiometric supramolecular tandem assays for phosphatase and phytase enzymes
- PMID: 38318943
- PMCID: PMC10911839
- DOI: 10.1039/d3ob02014b
Fluorescent ratiometric supramolecular tandem assays for phosphatase and phytase enzymes
Abstract
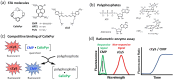
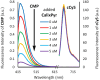
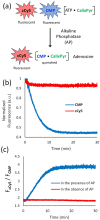
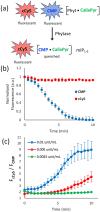
Ratiometric fluorescent assays have a built-in correction factor which enhances assay accuracy and reliability. We have developed fluorescent ratiometric supramolecular tandem assays for phosphatase and phytase enzymes using a mixture of three molecular components. One of the molecules is a tetra-cationic fluorescence quencher called CalixPyr which can bind and quench the polyanionic pyrene fluorophore, CMP, that emits at 430 nm. Polyphosphates can disrupt the CMP/CalixPyr complex and alter the fluorescence intensity (responsive signal). CalixPyr has no effect on the fluorescence emission of cationic pentamethine cyanine fluorophore, cCy5, which emits at 665 nm and acts as a non-responsive reference signal. The continuous ratiometric fluorescent assay for alkaline phosphatase monitored hydrolytic consumption of adenosine triphosphate (ATP). The continuous ratiometric fluorescent assay for phytase activity monitored hydrolytic consumption of phytate. With further development this latter assay may be useful for high throughput assessment of phytase activity in individual batches of fortified animal feed. It is likely that the three-molecule mixture (CMP, CalixPyr, cCy5) can become a general assay platform for other enzymes that catalyse addition/removal of phosphate groups from appropriate molecular substrates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Qi Y. L. Li Y. Z. Tan M. J. Yuan F. F. Murthy N. Duan Y. T. Zhu H. L. Yang S. Y. Coord. Chem. Rev. 2023;486:215130. doi: 10.1016/j.ccr.2023.215130. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

