A role for Vps13-mediated lipid transfer at the ER-endosome contact site in ESCRT-mediated sorting
- PMID: 38319250
- PMCID: PMC10847051
- DOI: 10.1083/jcb.202307094
A role for Vps13-mediated lipid transfer at the ER-endosome contact site in ESCRT-mediated sorting
Abstract
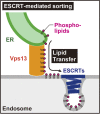
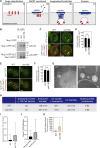
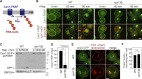
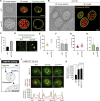
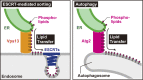
Endosomes are specialized organelles that function in the secretory and endocytic protein sorting pathways. Endocytosed cell surface receptors and transporters destined for lysosomal degradation are sorted into intraluminal vesicles (ILVs) at endosomes by endosomal sorting complexes required for transport (ESCRT) proteins. The endosomes (multivesicular bodies, MVBs) then fuse with the lysosome. During endosomal maturation, the number of ILVs increases, but the size of endosomes does not decrease despite the consumption of the limiting membrane during ILV formation. Vesicle-mediated trafficking is thought to provide lipids to support MVB biogenesis. However, we have uncovered an unexpected contribution of a large bridge-like lipid transfer protein, Vps13, in this process. Here, we reveal that Vps13-mediated lipid transfer at ER-endosome contact sites is required for the ESCRT pathway. We propose that Vps13 may play a critical role in supplying lipids to the endosome, ensuring continuous ESCRT-mediated sorting during MVB biogenesis.
© 2024 Suzuki et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

