The Hepatokine Orosomucoid 2 Mediates Beneficial Metabolic Effects of Bile Acids
- PMID: 38320268
- PMCID: PMC11043061
- DOI: 10.2337/db23-0520
The Hepatokine Orosomucoid 2 Mediates Beneficial Metabolic Effects of Bile Acids
Abstract
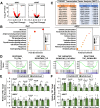
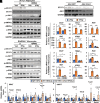
Bile acids (BAs) are pleiotropic regulators of metabolism. Elevated levels of hepatic and circulating BAs improve energy metabolism in peripheral organs, but the precise mechanisms underlying the metabolic benefits and harm still need to be fully understood. In the current study, we identified orosomucoid 2 (ORM2) as a liver-secreted hormone (i.e., hepatokine) induced by BAs and investigated its role in BA-induced metabolic improvements in mouse models of diet-induced obesity. Contrary to our expectation, under a high-fat diet (HFD), our Orm2 knockout (Orm2-KO) exhibited a lean phenotype compared with C57BL/6J control, partly due to the increased energy expenditure. However, when challenged with a HFD supplemented with cholic acid, Orm2-KO eliminated the antiobesity effect of BAs, indicating that ORM2 governs BA-induced metabolic improvements. Moreover, hepatic ORM2 overexpression partially replicated BA effects by enhancing insulin sensitivity. Mechanistically, ORM2 suppressed interferon-γ/STAT1 activities in inguinal white adipose tissue depots, forming the basis for anti-inflammatory effects of BAs and improving glucose homeostasis. In conclusion, our study provides new insights into the molecular mechanisms of BA-induced liver-adipose cross talk through ORM2 induction.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures





References
-
- Watanabe M, Houten SM, Mataki C, et al. . Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006;439:484–489 - PubMed
-
- Ippagunta SM, Kharitonenkov A, Adams AC, Hillgartner FB.. Cholic acid supplementation of a high-fat obesogenic diet suppresses hepatic triacylglycerol accumulation in mice via a fibroblast growth factor 21-dependent mechanism. J Nutr 2018;148:510–517 - PubMed
-
- Claudel T, Staels B, Kuipers F.. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol 2005;25:2020–2030 - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK125922/DK/NIDDK NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R56 DK128098/DK/NIDDK NIH HHS/United States
- R01 DK126656/DK/NIDDK NIH HHS/United States
- R01 DK122708/DK/NIDDK NIH HHS/United States
- R01 DK114356/DK/NIDDK NIH HHS/United States
- R01 DK121330/DK/NIDDK NIH HHS/United States
- R01HL154720/HL/NHLBI NIH HHS/United States
- P01 DK113954/DK/NIDDK NIH HHS/United States
- R01 DK122796/DK/NIDDK NIH HHS/United States
- P30CA125123/CA/NCI NIH HHS/United States
- R01 HL154720/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

