Anti-herbivory defences delivered by Epichloë fungal endophytes: a quantitative review of alkaloid concentration variation among hosts and plant parts
- PMID: 38320313
- PMCID: PMC11037487
- DOI: 10.1093/aob/mcae014
Anti-herbivory defences delivered by Epichloë fungal endophytes: a quantitative review of alkaloid concentration variation among hosts and plant parts
Abstract
Background and aims: In the subfamily Poöideae (Poaceae), certain grass species possess anti-herbivore alkaloids synthesized by fungal endophytes that belong to the genus Epichloë (Clavicipitaceae). The protective role of these symbiotic endophytes can vary, depending on alkaloid concentrations within specific plant-endophyte associations and plant parts.
Methods: We conducted a literature review to identify articles containing alkaloid concentration data for various plant parts in six important pasture species, Lolium arundinaceum, Lolium perenne, Lolium pratense, Lolium multiflorum|Lolium rigidum and Festuca rubra, associated with their common endophytes. We considered the alkaloids lolines (1-aminopyrrolizidines), peramine (pyrrolopyrazines), ergovaline (ergot alkaloids) and lolitrem B (indole-diterpenes). While all these alkaloids have shown bioactivity against insect herbivores, ergovaline and lolitrem B are harmful for mammals.
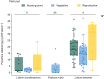
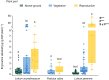
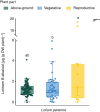
Key results: Loline alkaloid levels were higher in the perennial grasses L. pratense and L. arundinaceum compared to the annual species L. multiflorum and L. rigidum, and higher in reproductive tissues than in vegetative structures. This is probably due to the greater biomass accumulation in perennial species that can result in higher endophyte mycelial biomass. Peramine concentrations were higher in L. perenne than in L. arundinaceum and not affected by plant part. This can be attributed to the high within-plant mobility of peramine. Ergovaline and lolitrem B, both hydrophobic compounds, were associated with plant parts where fungal mycelium is usually present, and their concentrations were higher in plant reproductive tissues. Only loline alkaloid data were sufficient for below-ground tissue analyses and concentrations were lower than in above-ground parts.
Conclusions: Our study provides a comprehensive synthesis of fungal alkaloid variation across host grasses and plant parts, essential for understanding the endophyte-conferred defence extent. The patterns can be understood by considering endophyte growth within the plant and alkaloid mobility. Our study identifies research gaps, including the limited documentation of alkaloid presence in roots and the need to investigate the influence of different environmental conditions.
Keywords: Symbiosis; defensive mutualism; grass; herbivory resistance; plant–endophyte interaction; plant–herbivore interaction; secondary metabolites.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen J-P.. 2012. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338: 113–116. - PubMed
-
- Azevedo MD, Welty RE.. 1995. A study of the fungal endophyte Acremonium coenophialum in the roots of tall fescue seedlings. Mycologia 87: 289–297.
-
- Bacetty AA, Snook ME, Glenn AE, et al. 2009. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scribneri. Phytopathology 99: 1336–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

