Synaptic promiscuity in brain development
- PMID: 38320473
- PMCID: PMC10849093
- DOI: 10.1016/j.cub.2023.12.037
Synaptic promiscuity in brain development
Abstract
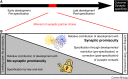
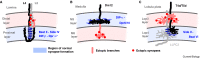
Precise synaptic connectivity is a prerequisite for the function of neural circuits, yet individual neurons, taken out of their developmental context, readily form unspecific synapses. How does the genome encode brain wiring in light of this apparent contradiction? Synaptic specificity is the outcome of a long series of developmental processes and mechanisms before, during and after synapse formation. How much promiscuity is permissible or necessary at the moment of synaptic partner choice depends on the extent to which prior development restricts available partners or subsequent development corrects initially made synapses. Synaptic promiscuity at the moment of choice can thereby play important roles in the development of precise connectivity, but also facilitate developmental flexibility and robustness. In this review, we assess the experimental evidence for the prevalence and roles of promiscuous synapse formation during brain development. Many well-established experimental approaches are based on developmental genetic perturbation and an assessment of synaptic connectivity only in the adult; this can make it difficult to pinpoint when a given defect or mechanism occurred. In many cases, such studies reveal mechanisms that restrict partner availability already prior to synapse formation. Subsequently, at the moment of choice, factors including synaptic competency, interaction dynamics and molecular recognition further restrict synaptic partners. The discussion of the development of synaptic specificity through the lens of synaptic promiscuity suggests an algorithmic process based on neurons capable of promiscuous synapse formation that are continuously prevented from making the wrong choices, with no single mechanism or developmental time point sufficient to explain the outcome.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Synapse formation in developing neural circuits.Curr Top Dev Biol. 2009;87:53-79. doi: 10.1016/S0070-2153(09)01202-2. Curr Top Dev Biol. 2009. PMID: 19427516 Free PMC article. Review.
-
Autophagy-dependent filopodial kinetics restrict synaptic partner choice during Drosophila brain wiring.Nat Commun. 2020 Mar 12;11(1):1325. doi: 10.1038/s41467-020-14781-4. Nat Commun. 2020. PMID: 32165611 Free PMC article.
-
Neuronal strategies for meeting the right partner during brain wiring.Curr Opin Neurobiol. 2020 Aug;63:1-8. doi: 10.1016/j.conb.2020.01.002. Epub 2020 Feb 6. Curr Opin Neurobiol. 2020. PMID: 32036252 Free PMC article. Review.
-
Autophagy in synapse formation and brain wiring.Autophagy. 2023 Oct;19(10):2814-2816. doi: 10.1080/15548627.2023.2179778. Epub 2023 Mar 2. Autophagy. 2023. PMID: 36779622 Free PMC article.
-
Microglia regulate synaptic development and plasticity.Dev Neurobiol. 2021 Jul;81(5):568-590. doi: 10.1002/dneu.22814. Epub 2021 Mar 8. Dev Neurobiol. 2021. PMID: 33583110 Free PMC article. Review.
Cited by
-
Spatial constraints and cell surface molecule depletion structure a randomly connected learning circuit.bioRxiv [Preprint]. 2024 Jul 21:2024.07.17.603956. doi: 10.1101/2024.07.17.603956. bioRxiv. 2024. Update in: Curr Biol. 2025 Jul 7;35(13):3191-3208.e10. doi: 10.1016/j.cub.2025.05.062. PMID: 39071296 Free PMC article. Updated. Preprint.
-
Nervous system-wide analysis of all C. elegans cadherins reveals neuron-specific functions across multiple anatomical scales.Sci Adv. 2025 Feb 21;11(8):eads2852. doi: 10.1126/sciadv.ads2852. Epub 2025 Feb 21. Sci Adv. 2025. PMID: 39983000 Free PMC article.
-
GLP-1/GLP-1R axis: from metabolism (obesity and T2DM) to immunity.Open Biol. 2025 Jan;15(7):240303. doi: 10.1098/rsob.240303. Epub 2025 Jul 2. Open Biol. 2025. PMID: 40592472 Free PMC article. Review.
-
Expanding ligand-receptor interaction networks for axon guidance: Structural insights into signal crosstalk and specificity.Curr Opin Neurobiol. 2025 Jun;92:102999. doi: 10.1016/j.conb.2025.102999. Epub 2025 Mar 20. Curr Opin Neurobiol. 2025. PMID: 40117944 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

