Immunogenicity of 2 or 3 Doses of 9vHPV Vaccine in U.S. Female Individuals 15 to 26 Years of Age
- PMID: 38320488
- PMCID: PMC11771182
- DOI: 10.1056/EVIDoa2300194
Immunogenicity of 2 or 3 Doses of 9vHPV Vaccine in U.S. Female Individuals 15 to 26 Years of Age
Abstract
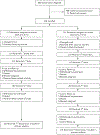
BACKGROUND: Within the United States, a 9-valent human papillomavirus (9vHPV) vaccine (HPV-6/11/16/18/31/33/45/52/58) is recommended as a two-dose series among individuals 9 to 14 years of age and a three-dose series among those 15 to 26 years of age. Data comparing two versus three doses of 9vHPV vaccine among individuals 15 to 26 years of age are limited. METHODS: We report on an ongoing, single-blinded, randomized noninferiority trial of the 9vHPV vaccine among individuals 15 to 26 years of age in the United States. Participants were randomly assigned to a two-dose (0 and 6 months) or three-dose (0, 2, and 6 months) schedule. Blood draws to assess antibody titers were planned before the first vaccination and at 1 and 6 months after the final vaccination. The primary outcome was the rate of seroconversion at 1 month after final vaccination. The secondary outcome was the two-dose versus three-dose ratio of antibody geometric mean titers (GMTs) for each of the 9vHPV genotypes at 1 and 6 months after final vaccination. This interim analysis reports results of female participants at 1 month after final vaccination. RESULTS: Of 860 participants screened, 438 were enrolled and randomly assigned to the two-dose (n=217) or three-dose (n=221) group. At 1 month after the final vaccine dose, the seroconversion rate for each of the nine HPV genotypes in the vaccine was 100% among participants in the two-dose group and 99% in the three-dose group. The point estimates of the two-dose versus three-dose ratios of antibody GMTs for eight of the nine HPV genotypes were above unity; the ratio for HPV-45 was 0.86 (95% confidence interval [CI], 0.66 to 1.13). This was also the smallest value for the lower bound of the 95% CI for all nine ratios (ratios above 1 favor the two-dose schedule). No serious adverse events were observed. CONCLUSIONS: In this unplanned interim analysis of U.S. female participants 15 to 26 years of age, two doses of 9vHPV vaccine appear to elicit responses similar to three doses at 1 month postvaccination. We await final results at 6 months following the last vaccine dose. (ClinicalTrials.gov number, NCT03943875.)
Figures
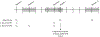
References
-
- zur Hausen H. Papillomavirus infections — a major cause of human cancers. Biochim Biophys Acta 1996;1288:F55–F78. - PubMed
-
- Priyadarshini M, Prabhu VS, Snedecor SJ, et al. Economic value of lost productivity attributable to human papillomavirus cancer mortality in the United States [published correction appears in Front Public Health 2021;9:691634]. Front Public Health 2021;8:624092. DOI: 10.3389/fpubh.2020.624092. - DOI - PMC - PubMed
-
- Yang S. Clinical review (STN 125508/0) - GARDASIL 9. Food and Drug Administration, 2014. (https://www.aimsib.org/wp-content/uploads/2018/12/clinical-review-stn-12...).
-
- Centers for Disease Control and Prevention. Cancers caused by HPV are preventable. November 1, 2021. (https://www.cdc.gov/hpv/hcp/protecting-patients.html).
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
