Targeted Inhibition of CYP11A1 in Castration-Resistant Prostate Cancer
- PMID: 38320513
- PMCID: PMC10852404
- DOI: 10.1056/EVIDoa2300171
Targeted Inhibition of CYP11A1 in Castration-Resistant Prostate Cancer
Erratum in
-
Targeted Inhibition of CYP11A1 in Castration-Resistant Prostate Cancer.NEJM Evid. 2024 Feb;3(2):EVIDx2300368. doi: 10.1056/EVIDx2300368. Epub 2024 Jan 3. NEJM Evid. 2024. PMID: 38349784
Abstract
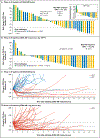
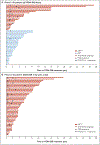
BACKGROUND: Prostate cancer is regulated by steroid hormones, even in castration-resistant disease. ODM-208, a novel inhibitor of cytochrome P450 11A1 (which catalyzes the first step of steroid-hormone biosynthesis), was investigated in patients with heavily pretreated metastatic castration-resistant prostate cancer (mCRPC). METHODS: CYPIDES is a first-in-human phase 1 (3 + 3 design) and phase 2 study. We administered ODM-208 twice daily with glucocorticoid/mineralocorticoid replacement and ongoing androgen deprivation therapy to adults with previously treated mCRPC, regardless of androgen receptor gene (AR) ligand-binding domain mutations (phase 1) and with activating AR ligand-binding domain mutations (ARmut; phase 2). Safety, pharmacokinetics, steroid-hormone pharmacodynamics, and preliminary efficacy were the key outcomes. RESULTS: Ninety-two patients received one or more doses of ODM-208: 47 in phase 1 (20 [42.6%] with ARmut) and 45 in phase 2 (all ARmut). A dose of ODM-208 of 5 mg twice a day with dexamethasone 1 mg/fludrocortisone 0.1 mg provided a balance between decreased steroidogenesis and toxicity. Treatment-related adrenal insufficiency was the most common toxicity in phase 1 (n=17, 36.2%; necessitating ODM-208 discontinuation in one patient); this toxicity occurred in six patients (13.3%) at 5 mg twice a day in phase 2. Median circulating testosterone levels declined from 3.0 ng/dl (interquartile range, 1.3 to 6.2 ng/dl) at baseline to undetectable levels within the first week of ODM-208 5 mg twice a day treatment in 46 of 53 (87%) patients. A decrease in prostate-specific antigen levels of 50% or more occurred in 14 of 19 (73.7%) patients with ARmut and 2 of 23 (8.7%) patients with AR wild type in phase 1 and in 24 of 45 (53.3%) patients with ARmut in phase 2. CONCLUSIONS: ODM-208 potently inhibited steroid-hormone biosynthesis with the expected toxicity of adrenal insufficiency. Evidence of antitumor activity was observed in this heavily pretreated mCRPC population, especially in those with ARmut. (Funded by Orion Pharma; ClinicalTrials.gov number, NCT03436485.)
Figures

References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): prostate cancer. January 2023. (https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf).
-
- Mottet N, Cornford P, Van den Bergh RCN, et al. EAU–EANM– ESTRO–ESUR–ISUP–SIPG guidelines on prostate cancer. March 2022. (https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-E...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
