A revised conceptual framework for mouse vomeronasal pumping and stimulus sampling
- PMID: 38320553
- PMCID: PMC10965388
- DOI: 10.1016/j.cub.2024.01.036
A revised conceptual framework for mouse vomeronasal pumping and stimulus sampling
Abstract
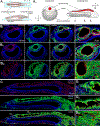
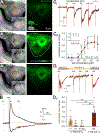
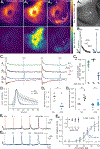
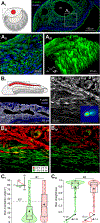
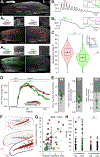
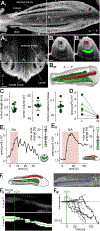
The physiological performance of any sensory organ is determined by its anatomy and physical properties. Consequently, complex sensory structures with elaborate features have evolved to optimize stimulus detection. Understanding these structures and their physical nature forms the basis for mechanistic insights into sensory function. Despite its crucial role as a sensor for pheromones and other behaviorally instructive chemical cues, the vomeronasal organ (VNO) remains a poorly characterized mammalian sensory structure. Fundamental principles of its physico-mechanical function, including basic aspects of stimulus sampling, remain poorly explored. Here, we revisit the classical vasomotor pump hypothesis of vomeronasal stimulus uptake. Using advanced anatomical, histological, and physiological methods, we demonstrate that large parts of the lateral mouse VNO are composed of smooth muscle. Vomeronasal smooth muscle tissue comprises two subsets of fibers with distinct topography, structure, excitation-contraction coupling, and, ultimately, contractile properties. Specifically, contractions of a large population of noradrenaline-sensitive cells mediate both transverse and longitudinal lumen expansion, whereas cholinergic stimulation targets an adluminal group of smooth muscle fibers. The latter run parallel to the VNO's rostro-caudal axis and are ideally situated to mediate antagonistic longitudinal constriction of the lumen. This newly discovered arrangement implies a novel mode of function. Single-cell transcriptomics and pharmacological profiling reveal the receptor subtypes involved. Finally, 2D/3D tomography provides non-invasive insight into the intact VNO's anatomy and mechanics, enables measurement of luminal fluid volume, and allows an assessment of relative volume change upon noradrenergic stimulation. Together, we propose a revised conceptual framework for mouse vomeronasal pumping and, thus, stimulus sampling.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
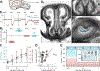
Comment in
-
Sensory neurobiology: Muscles power pheromone sensation.Curr Biol. 2024 Mar 25;34(6):R257-R259. doi: 10.1016/j.cub.2024.02.020. Curr Biol. 2024. PMID: 38531322
Similar articles
-
Deciphering the chemical language of inbred and wild mouse conspecific scents.Elife. 2024 May 15;12:RP90529. doi: 10.7554/eLife.90529. Elife. 2024. PMID: 38747258 Free PMC article.
-
Morphological and immunohistochemical study of the rabbit vomeronasal organ.J Anat. 2018 Dec;233(6):814-827. doi: 10.1111/joa.12884. Epub 2018 Sep 25. J Anat. 2018. PMID: 30255591 Free PMC article.
-
Imaging pheromone sensing in a mouse vomeronasal acute tissue slice preparation.J Vis Exp. 2011 Dec 6;(58):3311. doi: 10.3791/3311. J Vis Exp. 2011. PMID: 22157638 Free PMC article.
-
Pheromone detection by mammalian vomeronasal neurons.Microsc Res Tech. 2002 Aug 1;58(3):251-60. doi: 10.1002/jemt.10152. Microsc Res Tech. 2002. PMID: 12203702 Review.
-
Sensory coding of pheromone signals in mammals.Curr Opin Neurobiol. 2000 Aug;10(4):511-8. doi: 10.1016/s0959-4388(00)00121-5. Curr Opin Neurobiol. 2000. PMID: 10981622 Review.
Cited by
-
Noradrenaline modulates sensory information in mouse vomeronasal sensory neurons.iScience. 2024 Sep 2;27(10):110872. doi: 10.1016/j.isci.2024.110872. eCollection 2024 Oct 18. iScience. 2024. PMID: 39328934 Free PMC article.
-
Phosphodiesterase 5A regulates the vomeronasal pump in mice.Genesis. 2024 Jun;62(3):e23603. doi: 10.1002/dvg.23603. Genesis. 2024. PMID: 38738564 Free PMC article.
-
Ca2+-Activated Ion Channels Exert Opposite Effects in Different Signaling Compartments of Vomeronasal Sensory Neurons.J Neurosci. 2025 Apr 16;45(16):e2134242025. doi: 10.1523/JNEUROSCI.2134-24.2025. J Neurosci. 2025. PMID: 40032527 Free PMC article.
-
Following the p63/Keratin5 basal cells in the sensory and non-sensory epithelia of the vomeronasal organ.Genesis. 2024 Apr;62(2):e23596. doi: 10.1002/dvg.23596. Genesis. 2024. PMID: 38665067 Free PMC article.
-
Deciphering the chemical language of inbred and wild mouse conspecific scents.Elife. 2024 May 15;12:RP90529. doi: 10.7554/eLife.90529. Elife. 2024. PMID: 38747258 Free PMC article.
References
-
- Shepherd GM (2012). Neurogastronomy: How the Brain Creates Flavor and Why It Matters (Columbia University Press; ).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

