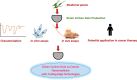
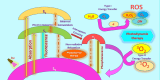
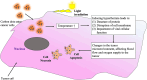
The future of plant based green carbon dots as cancer Nanomedicine: From current progress to future Perspectives and beyond
- PMID: 38320729
- PMCID: PMC11725112
- DOI: 10.1016/j.jare.2024.01.034
The future of plant based green carbon dots as cancer Nanomedicine: From current progress to future Perspectives and beyond
Abstract
Background: The emergence of carbon dots (CDs) as anticancer agents had sparked a transformation in cancer research and treatment strategies. These fluorescent CDs, initially introduced in the early 2000 s, possess exceptional biocompatibility, tunable fluorescence, and surface modification capabilities, positioning them as promising tools in biomedical applications.
Aim of review: The review encapsulates the transformative trajectory of green CDs as future anticancer nanomedicine, poised to redefine the strategies employed in the ongoing fight against cancer.
Key scientific concepts of review: The versatility of CDs was rooted in their various synthesis approaches and sustainable strategies, enabling their adaptability for diverse therapeutic uses. In vitro studies had showcased CDs' selective cytotoxicity against cancer cells while sparing healthy counterparts, forming the basis for targeted therapeutic potential. This selectivity had been attributed to the reactive oxygen species (ROS) generation, which opened avenues for targeted interventions. The role of CDs in combination therapies, synergizing with chemotherapy, radiotherapy, and targeted approaches was then investigated to heighten their anticancer efficacy. Notably, in vivo studies highlight CDs' remarkable biocompatibility and minimal side effects, endorsing their translational promise. Integration with conventional cancer treatments such as chemotherapy, radiotherapy, and immunotherapy amplified the versatility and effectiveness of CDs. The exploration of CDs' applications in photo-induced treatments further solidified their significance, positioning them as photosensitizers (PS) in photodynamic therapy (PDT) and photothermal agents (PA) in photothermal therapy (PTT). In PDT, CDs triggered the generation of ROS upon light exposure, facilitating cancer cell elimination, while in PTT, they induced localized hyperthermia within cancer cells, enhancing therapeutic outcomes. In vitro and in vivo investigations validated CDs' efficacy in PDT and PTT, affirming their potential for integration into combination therapies. Looking ahead, the future of CDs in anticancer treatment encompasses bioavailability, biocompatibility, synergistic treatments, tumor targeting, artificial intelligence (AI) and robotics integration, personalized medicine, and clinical translation. This transformative odyssey of CDs as future anticancer agents is poised to redefine the paradigm of cancer treatment strategies.
Keywords: Anticancer; Biocompatibility; Carbon Dots; Cytotoxicity; Photo-induced Treatment.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Surface-Modified Carbon Dots for Cancer Therapy: Integrating Diagnostic and Therapeutic Applications.Int J Nanomedicine. 2025 Jun 16;20:7715-7741. doi: 10.2147/IJN.S508181. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40546803 Free PMC article. Review.
-
Small Warriors of Nature: Novel Red Emissive Chlorophyllin Carbon Dots Harnessing Fenton-Fueled Ferroptosis for In Vitro and In Vivo Cancer Treatment.Small. 2024 May;20(18):e2309283. doi: 10.1002/smll.202309283. Epub 2024 Jan 17. Small. 2024. PMID: 38230862
-
Medicinal Plants Derived Green Carbon Dots: Synthesis, Characterization and Their Potential Applications in Cancer Therapy.Asian Pac J Cancer Prev. 2024 Oct 1;25(10):3393-3411. doi: 10.31557/APJCP.2024.25.10.3393. Asian Pac J Cancer Prev. 2024. PMID: 39471005 Free PMC article. Review.
-
Carbon Dots in Photodynamic Therapy: The Role of Dopant and Solvent on Optical and Photo-Responsive Properties.Chemistry. 2024 Sep 25;30(54):e202400885. doi: 10.1002/chem.202400885. Epub 2024 Sep 9. Chemistry. 2024. PMID: 39032088 Review.
-
Nucleolus-Targeting Carbon Dot Nanocomplexes for Combined Photodynamic/Photothermal Therapy.Mol Pharm. 2025 Feb 3;22(2):958-971. doi: 10.1021/acs.molpharmaceut.4c01211. Epub 2024 Dec 30. Mol Pharm. 2025. PMID: 39895310
Cited by
-
Effect of temperature on the carbonization process of cationic carbon dots: a physicochemical and in vitro study.RSC Adv. 2025 Apr 28;15(16):12814-12824. doi: 10.1039/d5ra00062a. eCollection 2025 Apr 16. RSC Adv. 2025. PMID: 40297713 Free PMC article.
-
Theranostic Applications of Taurine-Derived Carbon Dots in Colorectal Cancer: Ferroptosis Induction and Multifaceted Antitumor Mechanisms.Int J Nanomedicine. 2025 Jun 16;20:7613-7635. doi: 10.2147/IJN.S516926. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40546797 Free PMC article.
-
Applications of Green Carbon Dots in Personalized Diagnostics for Precision Medicine.Int J Mol Sci. 2025 Mar 21;26(7):2846. doi: 10.3390/ijms26072846. Int J Mol Sci. 2025. PMID: 40243410 Free PMC article. Review.
-
Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota.Int J Mol Sci. 2025 Jun 6;26(12):5433. doi: 10.3390/ijms26125433. Int J Mol Sci. 2025. PMID: 40564897 Free PMC article. Review.
References
-
- Mohan G, Ayisha Hamna T.P., Jijo A.J., Saradha Devi K.M., Narayanasamy A., Vellingiri B. Recent advances in radiotherapy and its associated side effects in cancer—A review. J Basic Appl Zoology 2019;80:14. 10.1186/s41936-019-0083-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials