Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis
- PMID: 38320981
- PMCID: PMC10847061
- DOI: 10.1002/jev2.12410
Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis
Erratum in
-
Correction to "Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis".J Extracell Vesicles. 2024 Apr;13(4):e12441. doi: 10.1002/jev2.12441. J Extracell Vesicles. 2024. PMID: 38647128 Free PMC article. No abstract available.
-
Correction to article pagination in the Journal of Extracellular Vesicles.J Extracell Vesicles. 2024 May;13(5):e12443. doi: 10.1002/jev2.12443. J Extracell Vesicles. 2024. PMID: 38695388 Free PMC article. No abstract available.
Abstract
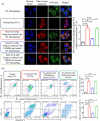
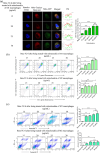
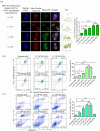
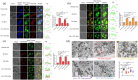
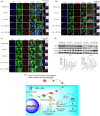
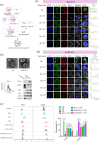
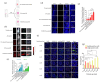
Extracellular vesicles (EVs) exert a significant influence not only on the pathogenesis of diseases but also on their therapeutic interventions, contingent upon the variances observed in their originating cells. Mitochondria can be transported between cells via EVs to promote pathological changes. In this study, we found that EVs derived from M1 macrophages (M1-EVs), which encapsulate inflammatory mitochondria, can penetrate pancreatic beta cells. Inflammatory mitochondria fuse with the mitochondria of pancreatic beta cells, resulting in lipid peroxidation and mitochondrial disruption. Furthermore, fragments of mitochondrial DNA (mtDNA) are released into the cytosol, activating the STING pathway and ultimately inducing apoptosis. The potential of adipose-derived stem cell (ADSC)-released EVs in suppressing M1 macrophage reactions shows promise. Subsequently, ADSC-EVs were utilized and modified with an F4/80 antibody to specifically target macrophages, aiming to treat ferroptosis of pancreatic beta cells in vivo. In summary, our data further demonstrate that EVs secreted from M1 phenotype macrophages play major roles in beta cell ferroptosis, and the modified ADSC-EVs exhibit considerable potential for development as a vehicle for targeted delivery to macrophages.
Keywords: M1 macrophages; extracellular vesicles; ferroptosis; mitochondria; pancreatic beta cells.
© 2024 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Alonso‐Herranz, L. , Porcuna, J. , & Ricote, M. (2019). Isolation and purification of tissue resident macrophages for the analysis of nuclear receptor activity. Methods in Molecular Biology, 1951, 59–73. - PubMed
-
- Chen, J. , Fu, C. Y. , Shen, G. , Wang, J. , Xu, L. , Li, H. , Cao, X. , Zheng, M.‐Z. , Shen, Y.‐L. , Zhong, J. , Chen, Y.‐Y. , & Wang, L. L. (2022a). Macrophages induce cardiomyocyte ferroptosis via mitochondrial transfer. Free Radical Biology and Medicine, 190, 1–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

