Colloidal Aziridinium Lead Bromide Quantum Dots
- PMID: 38320982
- PMCID: PMC10883123
- DOI: 10.1021/acsnano.3c11579
Colloidal Aziridinium Lead Bromide Quantum Dots
Abstract
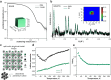
The compositional engineering of lead-halide perovskite nanocrystals (NCs) via the A-site cation represents a lever to fine-tune their structural and electronic properties. However, the presently available chemical space remains minimal since, thus far, only three A-site cations have been reported to favor the formation of stable lead-halide perovskite NCs, i.e., Cs+, formamidinium (FA), and methylammonium (MA). Inspired by recent reports on bulk single crystals with aziridinium (AZ) as the A-site cation, we present a facile colloidal synthesis of AZPbBr3 NCs with a narrow size distribution and size tunability down to 4 nm, producing quantum dots (QDs) in the regime of strong quantum confinement. NMR and Raman spectroscopies confirm the stabilization of the AZ cations in the locally distorted cubic structure. AZPbBr3 QDs exhibit bright photoluminescence with quantum efficiencies of up to 80%. Stabilized with cationic and zwitterionic capping ligands, single AZPbBr3 QDs exhibit stable single-photon emission, which is another essential attribute of QDs. In particular, didodecyldimethylammonium bromide and 2-octyldodecyl-phosphoethanolamine ligands afford AZPbBr3 QDs with high spectral stability at both room and cryogenic temperatures, reduced blinking with a characteristic ON fraction larger than 85%, and high single-photon purity (g(2)(0) = 0.1), all comparable to the best-reported values for MAPbBr3 and FAPbBr3 QDs of the same size.
Keywords: aziridinium; ligands; nanocrystals; perovskite; photoluminescence; quantum dots.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. - DOI - PMC - PubMed
-
- Rainò G.; Nedelcu G.; Protesescu L.; Bodnarchuk M. I.; Kovalenko M. V.; Mahrt R. F.; Stöferle T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single-Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10, 2485–2490. 10.1021/acsnano.5b07328. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

