Sulbactam protects neurons against double neurotoxicity of amyloid beta and glutamate load by upregulating glial glutamate transporter 1
- PMID: 38320997
- PMCID: PMC10847450
- DOI: 10.1038/s41420-024-01827-5
Sulbactam protects neurons against double neurotoxicity of amyloid beta and glutamate load by upregulating glial glutamate transporter 1
Abstract
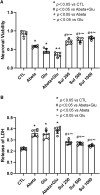
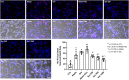
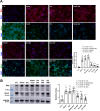
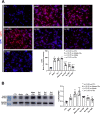
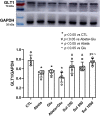
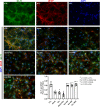
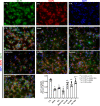
Amyloid beta (Abeta) synergistically enhances excitotoxicity of glutamate load by impairing glutamate transporter 1 (GLT1) expression and function, which exacerbates the development of Alzheimer's disease (AD). Our previous studies suggested that sulbactam can upregulate the expression levels and capacity of GLT1. Therefore, this study aims to investigate whether sulbactam improves neuronal tolerance against neurotoxicity of Abeta and glutamate load by up-regulating GLT1 in primary neuron-astrocyte co-cultures. Early postnatal P0-P1 Wistar rat pups' cortices were collected for primary neuron-astrocyte cultures. Hoechst-propidium iodide (HO-PI) stain and lactate dehydrogenase (LDH) assays were used to analyze neuronal death. Cell counting kit 8 (CCK8) was applied to determine cell viability. Immunofluorescence staining and western blotting were used to assess protein expressions including GLT1, B-cell lymphoma 2 (BCL2), BCL2 associated X (BAX), and cleaved caspase 3 (CCP3). Under the double effect of Abeta and glutamate load, more neurons were lost than that induced by Abeta or glutamate alone, shown as decreased cell viability, increased LDH concentration in the cultural medium, HO-PI positive stains, high CCP3 expression, and high BAX/BCL2 ratio resulting from increased BAX and decreased BCL2 expressions. Notably, pre-incubation with sulbactam significantly attenuated the neuronal loss and activation of apoptosis induced by both Abeta and glutamate in a dose-dependent manner. Simultaneously, both astrocytic and neuronal GLT1 expressions were upregulated after sulbactam incubation. Taken together, it could be concluded that sulbactam protected neurons against double neurotoxicity of Abeta and glutamate load by upregulating GLT1 expression. The conclusion provides evidence for potential intervention using sulbactam in AD research.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, et al. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–71. doi: 10.1016/j.neuroscience.2009.03.048. - DOI - PubMed
-
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta 1-42. J Neurochem. 2001;78:413–6. doi: 10.1046/j.1471-4159.2001.00451.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

