A phase 1/2 clinical trial of invariant natural killer T cell therapy in moderate-severe acute respiratory distress syndrome
- PMID: 38321023
- PMCID: PMC10847411
- DOI: 10.1038/s41467-024-44905-z
A phase 1/2 clinical trial of invariant natural killer T cell therapy in moderate-severe acute respiratory distress syndrome
Abstract
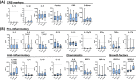
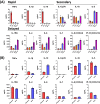
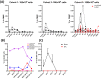
Invariant natural killer T (iNKT) cells, a unique T cell population, lend themselves for use as adoptive therapy due to diverse roles in orchestrating immune responses. Originally developed for use in cancer, agenT-797 is a donor-unrestricted allogeneic ex vivo expanded iNKT cell therapy. We conducted an open-label study in virally induced acute respiratory distress syndrome (ARDS) caused by the severe acute respiratory syndrome-2 virus (trial registration NCT04582201). Here we show that agenT-797 rescues exhausted T cells and rapidly activates both innate and adaptive immunity. In 21 ventilated patients including 5 individuals receiving veno-venous extracorporeal membrane oxygenation (VV-ECMO), there are no dose-limiting toxicities. We observe an anti-inflammatory systemic cytokine response and infused iNKT cells are persistent during follow-up, inducing only transient donor-specific antibodies. Clinical signals of associated survival and prevention of secondary infections are evident. Cellular therapy using off-the-shelf iNKT cells is safe, can be rapidly scaled and is associated with an anti-inflammatory response. The safety and therapeutic potential of iNKT cells across diseases including infections and cancer, warrants randomized-controlled trials.
© 2024. The Author(s).
Conflict of interest statement
M.A.P., S.K., D.M., B.Y., S.B., X.M., R.M., A.B., J.S.B., and M.v.D. are current or former employees of MiNK Therapeutics and have received stock and compensation. M.K., A.B., D.C., V.N., W.O., K.S., X.S., J.C., Y.Q. are current employees of Agenus Inc and have received stock and compensation. J.S. from 2020 has been the Editor-in-Chief of Oncogene has sat on SABs for Vaccitech, Heat Biologics, Eli Lilly, Alveo Technologies, Pear Bio, Agenus (and received compensation), Equilibre Biopharmaceuticals, Graviton Bioscience Corporation, Celltrion, Volvox, Certis, Greenmantle, vTv Therapeutics, APIM Therapeutics, Onconox, IO Labs, Bryologyx, Zephyr AI and Benevolent AI. He has consulted with Lansdowne partners and Vitruvian. He chairs the Board of Directors for Xerion and previously BB Biotech Healthcare Trust PLC. M.A.E. is former employee and current consultant for MiNK, employed by Imvax Inc., I.M. has received consulting fees from Agenus and TCH is on MiNK Therapeutics SAB and a consultant, has received Agenus research funding and consulting fees, and is on the Pfizer International Viral Advisory Council. J.R. received research funding from Equillium, Kite Pharma, Novartis and Oncternal and served on SABs for Akron Biotech, Avrobio, Clade Therapeutics, Erbi Biosystems, Garuda Therapeutics, LifeVault Bio, Novartis, Smart Immune, Talaris Therapeutics and TScan Therapeutics. A.G.A. is on the SAB for Legend Biotech, and a speaker for Bristol Myers Squibb. S.N. sits on the SABs for Kite/Gilead, Iovance, GlaxoSmithKline, Sobi, A2 Bio. K.v.B. has received stock and other ownership from Hemogeny, has a consultant role to HemOgenyx, Glycostem, Gamida Cell, CTI, Intellia, SNIPR BIOME, MorphoSys, Incyte, Autolus, ADC Therapeutics, has received research funding from Precision Biosciences, Orca Bio, Bristol-Myers Squibb/Celgene, Calibr and Actinium Pharmaceuticals. No other author declares a conflict.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

